Hypoxia-inducible factors in T lymphocyte differentiation and function. A Review in the Theme: Cellular Responses to Hypoxia
- PMID: 26354751
- PMCID: PMC4628938
- DOI: 10.1152/ajpcell.00204.2015
Hypoxia-inducible factors in T lymphocyte differentiation and function. A Review in the Theme: Cellular Responses to Hypoxia
Abstract
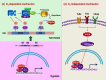
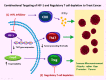
Low oxygen concentrations or hypoxia is a trait common to inflamed tissues. Therefore it is not surprising that pathways of hypoxic stress response, largely governed by hypoxia-inducible factors (HIF), are highly relevant to the proper function of immune cells. HIF expression and stabilization in immune cells can be triggered not only by hypoxia, but also by a variety of stimuli and pathological stresses associated with leukocyte activation and inflammation. In addition to its role as a sensor of oxygen scarcity, HIF is also a major regulator of immune cell metabolic function. Rapid progress is being made in elucidating the roles played by HIF in diverse aspects of both innate and adaptive immunity. Here we discuss a number of breakthroughs that have shed light on how HIF expression and activity impact the differentiation and function of diverse T cell populations. The insights gained from these findings may serve as the foundation for future therapies aimed at fine-tuning the immune response.
Keywords: T cells; hypoxia-inducible factor; immune activation; inflammation; metabolism.
Copyright © 2015 the American Physiological Society.
Figures
References
-
- Ben-Shoshan J, Maysel-Auslender S, Mor A, Keren G, George J. Hypoxia controls CD4+CD25+ regulatory T-cell homeostasis via hypoxia-inducible factor-1alpha. Eur J Immunol 38: 2412–2418, 2008. - PubMed
-
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441: 235–238, 2006. - PubMed
-
- Blouin CC, Page EL, Soucy GM, Richard DE. Hypoxic gene activation by lipopolysaccharide in macrophages: implication of hypoxia-inducible factor 1alpha. Blood 103: 1124–1130, 2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

