Complement deposition in autoimmune hemolytic anemia is a footprint for difficult-to-detect IgM autoantibodies
- PMID: 26354757
- PMCID: PMC4825291
- DOI: 10.3324/haematol.2015.128991
Complement deposition in autoimmune hemolytic anemia is a footprint for difficult-to-detect IgM autoantibodies
Abstract
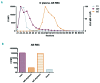
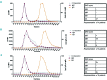
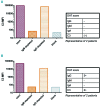
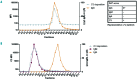
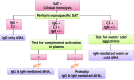
In autoimmune hemolytic anemia autoantibodies against erythrocytes lead to increased clearance of the erythrocytes, which in turn results in a potentially fatal hemolytic anemia. Depending on whether IgG or IgM antibodies are involved, response to therapy is different. Proper identification of the isotype of the anti-erythrocyte autoantibodies is, therefore, crucial. However, detection of IgM autoantibodies can be challenging. We, therefore, set out to improve the detection of anti-erythrocyte IgM. Direct detection using a flow cytometry-based approach did not yield satisfactory improvements. Next, we analyzed whether the presence of complement C3 on a patient's erythrocytes could be used for indirect detection of anti-erythrocyte IgM. To this end, we fractionated patients' sera by size exclusion chromatography and tested which fractions yielded complement deposition on erythrocytes. Strikingly, we found that all patients with C3 on their erythrocytes according to standard diagnostic tests had an IgM anti-erythrocyte component that could activate complement, even if no such autoantibody had been detected with any other test. This also included all tested patients with only IgG and C3 on their erythrocytes, who would previously have been classified as having an IgG-only mediated autoimmune hemolytic anemia. Depleting patients' sera of either IgG or IgM and testing the remaining complement activation confirmed this result. In conclusion, complement activation in autoimmune hemolytic anemia is mostly IgM-mediated and the presence of covalent C3 on patients' erythrocytes can be taken as a footprint of the presence of anti-erythrocyte IgM. Based on this finding, we propose a diagnostic workflow that will aid in choosing the optimal treatment strategy.
Copyright© Ferrata Storti Foundation.
Figures

References
-
- Petz LD, Garratty G, Immune hemolytic anemia’s. Chapter 3: Classification and clinical characteristics of autoimmune hemolytic anemias. Churchill Livingstone, Philadelphia, Pennsylvania, USA, 1980; 2nd edition: 61–114.
-
- Barros MM, Blajchman MA, Bordin JO. Warm autoimmune hemolytic anemia: recent progress in understanding the immunobiology and the treatment. Transfus Med Rev 2010;24(3):195–210. - PubMed
-
- Petz LD. Cold antibody autoimmune hemolytic anemias. Blood Rev 2008;22(1): 1–15. - PubMed
-
- Engelfriet CP, van’t Veer MB, Maas N, Ouwehand WH, Beckers DO, von den Borne AE. Autoimmune haemolytic anemias. In: Kay AB, Denman AM, Wright R, eds. Clinical Immunology and Allergy. London: Baillieres Tindall; 1987:251–267.
-
- Zeerleder S. Autoimmune haemolytic anaemia – a practical guide to cope with a diagnostic and therapeutic challenge. Neth J Med 2011;69(4):177–184. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

