Covalency-reinforced oxygen evolution reaction catalyst
- PMID: 26354832
- PMCID: PMC4579779
- DOI: 10.1038/ncomms9249
Covalency-reinforced oxygen evolution reaction catalyst
Abstract
The oxygen evolution reaction that occurs during water oxidation is of considerable importance as an essential energy conversion reaction for rechargeable metal-air batteries and direct solar water splitting. Cost-efficient ABO3 perovskites have been studied extensively because of their high activity for the oxygen evolution reaction; however, they lack stability, and an effective solution to this problem has not yet been demonstrated. Here we report that the Fe(4+)-based quadruple perovskite CaCu3Fe4O12 has high activity, which is comparable to or exceeding those of state-of-the-art catalysts such as Ba(0.5)Sr(0.5)Co(0.8)Fe(0.2)O(3-δ) and the gold standard RuO2. The covalent bonding network incorporating multiple Cu(2+) and Fe(4+) transition metal ions significantly enhances the structural stability of CaCu3Fe4O12, which is key to achieving highly active long-life catalysts.
Figures

 for Mn3+ and Fe4+, respectively, where
for Mn3+ and Fe4+, respectively, where  denotes a ligand hole at the O 2p orbital. The π-bonds between the t2g and 2p orbitals are neglected for simplicity. (b) Crystal structures and 3D electron density maps of SFO and CCFO. SFO is crystallized in a cubic ABO3-type perovskite structure, and CCFO is crystallized in a cubic quadruple AA′3B4O12-type structure with a 2a0 × 2a0 × 2a0 unit cell (a0: a-axis length of a simple ABO3 perovskite). In these types of perovskites, the A-sites are occupied by alkaline, alkaline-earth or rare-earth metal ions, the A′-sites by Jahn–Teller active ions such as Cu2+ and Mn3+, and the B-sites by d-block transition metal ions. 3D electron density maps of SFO (equi-density level: 0.4 Å−1) and CCFO (equi-density level: 0.5 Å−1) were obtained from maximum entropy method analysis of synchrotron X-ray powder diffraction data. The shaded cross-sections indicate the (110) and
denotes a ligand hole at the O 2p orbital. The π-bonds between the t2g and 2p orbitals are neglected for simplicity. (b) Crystal structures and 3D electron density maps of SFO and CCFO. SFO is crystallized in a cubic ABO3-type perovskite structure, and CCFO is crystallized in a cubic quadruple AA′3B4O12-type structure with a 2a0 × 2a0 × 2a0 unit cell (a0: a-axis length of a simple ABO3 perovskite). In these types of perovskites, the A-sites are occupied by alkaline, alkaline-earth or rare-earth metal ions, the A′-sites by Jahn–Teller active ions such as Cu2+ and Mn3+, and the B-sites by d-block transition metal ions. 3D electron density maps of SFO (equi-density level: 0.4 Å−1) and CCFO (equi-density level: 0.5 Å−1) were obtained from maximum entropy method analysis of synchrotron X-ray powder diffraction data. The shaded cross-sections indicate the (110) and  planes of SFO and CCFO, respectively. The widespread covalent network incorporating the Cu, Fe and O ions is exemplified by CCFO. These illustrations were drawn using the VESTA3 program. The synchrotron X-ray powder diffraction patterns and Rietveld refinement results are shown in Supplementary Fig. 1 and Supplementary Note 1.
planes of SFO and CCFO, respectively. The widespread covalent network incorporating the Cu, Fe and O ions is exemplified by CCFO. These illustrations were drawn using the VESTA3 program. The synchrotron X-ray powder diffraction patterns and Rietveld refinement results are shown in Supplementary Fig. 1 and Supplementary Note 1.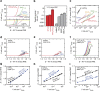
 and η=Eonset−1.23 (V). (b) Specific activities (current density at 1.6 V versus RHE) for SFO, CFO, CCFO, LMO, BSCF and RuO2. (c) Tafel plots for SFO, CFO, CCFO, LMO and BSCF. The error bars show the s.d. of three independent measurements. All data in (a–c) were obtained from the third cycle. Cyclic voltammograms of (d) SFO, (e) CFO and (f) CCFO for 100 cycles. Cycle dependence of Tafel slopes for (g) SFO, (h) CFO and (i) CCFO. Hundred continuous cycle measurements were performed with a higher disk rotation rate of 3,200 r.p.m. to prevent adhesion of the O2 bubbles to the electrode. In (b,c) the error bars correspond to the s.d. obtained from three independent measurements.
and η=Eonset−1.23 (V). (b) Specific activities (current density at 1.6 V versus RHE) for SFO, CFO, CCFO, LMO, BSCF and RuO2. (c) Tafel plots for SFO, CFO, CCFO, LMO and BSCF. The error bars show the s.d. of three independent measurements. All data in (a–c) were obtained from the third cycle. Cyclic voltammograms of (d) SFO, (e) CFO and (f) CCFO for 100 cycles. Cycle dependence of Tafel slopes for (g) SFO, (h) CFO and (i) CCFO. Hundred continuous cycle measurements were performed with a higher disk rotation rate of 3,200 r.p.m. to prevent adhesion of the O2 bubbles to the electrode. In (b,c) the error bars correspond to the s.d. obtained from three independent measurements.

References
-
- Fabbri E., Habereder A., Walter K., Kötz R. & Schmidt T. J. Developments and perspectives of oxide-based catalysts for the oxygen evolution reaction. Catal. Sci. Technol. 4, 3800–3821 (2014).
-
- Hong W. T. et al. Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energy Environ. Sci. 8, 1404–1427 (2015).
-
- Katsounaros I., Cherevko S., Zeradjanin A. R. & Mayrhofer K. J. J. Oxygen electrochemistry as a cornerstone for sustainable energy conversion. Angew. Chem. Int. Ed. 53, 102–121 (2014). - PubMed
-
- Wang Z. -L., Xu D., Xu J. -J. & Zhang X. -B. Oxygen electrocatalysts in metal-air batteries: from aqueous to nonaqueous electrolytes. Chem. Soc. Rev. 43, 7746–7786 (2014). - PubMed
-
- Subbaraman R. et al. Trends in activity for the water electrolyser reactions on 3d M(Ni,Co,Fe,Mn) hydr(oxy)oxide catalysts. Nat. Mater. 11, 550–557 (2012). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

