Neurotrophin-3 Enhances the Synaptic Organizing Function of TrkC-Protein Tyrosine Phosphatase σ in Rat Hippocampal Neurons
- PMID: 26354911
- PMCID: PMC4563035
- DOI: 10.1523/JNEUROSCI.1330-15.2015
Neurotrophin-3 Enhances the Synaptic Organizing Function of TrkC-Protein Tyrosine Phosphatase σ in Rat Hippocampal Neurons
Abstract
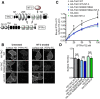
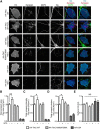
Neurotrophin-3 (NT-3) and its high-affinity receptor TrkC play crucial trophic roles in neuronal differentiation, axon outgrowth, and synapse development and plasticity in the nervous system. We demonstrated previously that postsynaptic TrkC functions as a glutamatergic synapse-inducing (synaptogenic) cell adhesion molecule trans-interacting with presynaptic protein tyrosine phosphatase σ (PTPσ). Given that NT-3 and PTPσ bind distinct domains of the TrkC extracellular region, here we tested the hypothesis that NT-3 modulates TrkC/PTPσ binding and synaptogenic activity. NT-3 enhanced PTPσ binding to cell surface-expressed TrkC and facilitated the presynapse-inducing activity of TrkC in rat hippocampal neurons. Imaging of recycling presynaptic vesicles combined with TrkC knockdown and rescue approaches demonstrated that NT-3 rapidly potentiates presynaptic function via binding endogenous postsynaptic TrkC in a tyrosine kinase-independent manner. Thus, NT-3 positively modulates the TrkC-PTPσ complex for glutamatergic presynaptic assembly and function independently from TrkC kinase activation. Our findings provide new insight into synaptic roles of neurotrophin signaling and mechanisms controlling synaptic organizing complexes. Significance statement: Although many synaptogenic adhesion complexes have been identified in recent years, little is known about modulatory mechanisms. Here, we demonstrate a novel role of neurotrophin-3 in synaptic assembly and function as a positive modulator of the TrkC-protein tyrosine phosphatase σ complex. This study provides new insight into the involvement of neurotrophin signaling in synapse development and plasticity, presenting a molecular mechanism that may underlie previous observations of short- and long-term enhancement of presynaptic function by neurotrophin. Given the links of synaptogenic adhesion molecules to autism and schizophrenia, this study might also contribute to a better understanding of the pathogenesis of these disorders and provide a new direction for ameliorating imbalances in synaptic signaling networks.
Keywords: PTPσ; TrkC; neurotrophin-3; synapse organization; synaptic plasticity.
Copyright © 2015 the authors 0270-6474/15/3512425-07$15.00/0.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
