Molecular classification of anaplastic oligodendroglioma using next-generation sequencing: a report of the prospective randomized EORTC Brain Tumor Group 26951 phase III trial
- PMID: 26354927
- PMCID: PMC4767239
- DOI: 10.1093/neuonc/nov182
Molecular classification of anaplastic oligodendroglioma using next-generation sequencing: a report of the prospective randomized EORTC Brain Tumor Group 26951 phase III trial
Abstract
Background: Histopathological diagnosis of diffuse gliomas is subject to interobserver variation and correlates modestly with major prognostic and predictive molecular abnormalities. We investigated a series of patients with locally diagnosed anaplastic oligodendroglial tumors included in the EORTC phase III trial 26951 on procarbazine/lomustine/vincristine (PCV) chemotherapy to explore the diagnostic, prognostic, and predictive value of targeted next-generation sequencing (NGS) in diffuse glioma and to assess the prognostic impact of FUBP1 and CIC mutations.
Methods: Mostly formalin-fixed paraffin-embedded samples were tested with targeted NGS for mutations in ATRX, TP53, IDH1, IDH2, CIC, FUBP1, PI3KC, TERT, EGFR, H3F3A, BRAF, PTEN, and NOTCH and for copy number alterations of chromosomes 1p, 19q, 10q, and 7. TERT mutations were also assessed, with PCR.
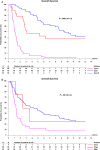
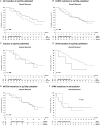
Results: Material was available from 139 cases, in 6 of which results were uninformative. One hundred twenty-six tumors could be classified: 20 as type II (IDH mutation [mut], "astrocytoma"), 49 as type I (1p/19q codeletion, "oligodendroglioma"), 55 as type III (7+/10q- or TERTmut and 1p/19q intact, "glioblastoma"), and 2 as childhood glioblastoma (H3F3Amut), leaving 7 unclassified (total 91% classified). Molecular classification was of clear prognostic significance and correlated better with outcome than did classical histopathology. In 1p/19q codeleted tumors, outcome was not affected by CIC and FUBP1 mutations. MGMT promoter methylation remained the most predictive factor for survival benefit of PCV chemotherapy.
Conclusion: Targeted NGS allows a clinically relevant classification of diffuse glioma into groups with very different outcomes. The diagnosis of diffuse glioma should be primarily based on a molecular classification, with the histopathological grade added to it. Future discussion should primarily aim at establishing the minimum requirements for molecular classification of diffuse glioma.
Keywords: 1p/19q; PCV; methylation; next generation sequencing; oligodendroglioma.
© The Author(s) 2015. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Kros JM, Gorlia T, Kouwenhoven MC, et al. Panel review of anaplastic oligodendroglioma from EORTC trial 26951: assessment of consensus in diagnosis, influence of 1p/19q loss and correlations with outcome. J Neuropathol Exp Neurol. 2007;66(6):545–551. - PubMed
-
- Wick W, Hartmann C, Engel C, et al. NOA-04 randomized phase iii trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27(35):5874–5880. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

