A Highly Immunogenic and Protective Middle East Respiratory Syndrome Coronavirus Vaccine Based on a Recombinant Measles Virus Vaccine Platform
- PMID: 26355094
- PMCID: PMC4645655
- DOI: 10.1128/JVI.01815-15
A Highly Immunogenic and Protective Middle East Respiratory Syndrome Coronavirus Vaccine Based on a Recombinant Measles Virus Vaccine Platform
Abstract
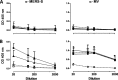
In 2012, the first cases of infection with the Middle East respiratory syndrome coronavirus (MERS-CoV) were identified. Since then, more than 1,000 cases of MERS-CoV infection have been confirmed; infection is typically associated with considerable morbidity and, in approximately 30% of cases, mortality. Currently, there is no protective vaccine available. Replication-competent recombinant measles virus (MV) expressing foreign antigens constitutes a promising tool to induce protective immunity against corresponding pathogens. Therefore, we generated MVs expressing the spike glycoprotein of MERS-CoV in its full-length (MERS-S) or a truncated, soluble variant of MERS-S (MERS-solS). The genes encoding MERS-S and MERS-solS were cloned into the vaccine strain MVvac2 genome, and the respective viruses were rescued (MVvac2-CoV-S and MVvac2-CoV-solS). These recombinant MVs were amplified and characterized at passages 3 and 10. The replication of MVvac2-CoV-S in Vero cells turned out to be comparable to that of the control virus MVvac2-GFP (encoding green fluorescent protein), while titers of MVvac2-CoV-solS were impaired approximately 3-fold. The genomic stability and expression of the inserted antigens were confirmed via sequencing of viral cDNA and immunoblot analysis. In vivo, immunization of type I interferon receptor-deficient (IFNAR(-/-))-CD46Ge mice with 2 × 10(5) 50% tissue culture infective doses of MVvac2-CoV-S(H) or MVvac2-CoV-solS(H) in a prime-boost regimen induced robust levels of both MV- and MERS-CoV-neutralizing antibodies. Additionally, induction of specific T cells was demonstrated by T cell proliferation, antigen-specific T cell cytotoxicity, and gamma interferon secretion after stimulation of splenocytes with MERS-CoV-S presented by murine dendritic cells. MERS-CoV challenge experiments indicated the protective capacity of these immune responses in vaccinated mice.
Importance: Although MERS-CoV has not yet acquired extensive distribution, being mainly confined to the Arabic and Korean peninsulas, it could adapt to spread more readily among humans and thereby become pandemic. Therefore, the development of a vaccine is mandatory. The integration of antigen-coding genes into recombinant MV resulting in coexpression of MV and foreign antigens can efficiently be achieved. Thus, in combination with the excellent safety profile of the MV vaccine, recombinant MV seems to constitute an ideal vaccine platform. The present study shows that a recombinant MV expressing MERS-S is genetically stable and induces strong humoral and cellular immunity against MERS-CoV in vaccinated mice. Subsequent challenge experiments indicated protection of vaccinated animals, illustrating the potential of MV as a vaccine platform with the potential to target emerging infections, such as MERS-CoV.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures




References
-
- World Health Organization. 2014. Middle East respiratory syndrome coronavirus (MERS-CoV)—Saudi Arabia. World Health Organization, Geneva, Switzerland: http://www.who.int/csr/don/17-december-2014-mers/en/.
-
- Drosten C, Meyer B, Müller MA, Corman VM, Al-Masri M, Hossain R, Madani H, Sieberg A, Bosch BJ, Lattwein E, Alhakeem RF, Assiri AM, Hajomar W, Albarrak AM, Al-Tawfiq JA, Zumla AI, Memish ZA. 2014. Transmission of MERS-coronavirus in household contacts. N Engl J Med 371:828–835. doi:10.1056/NEJMoa1405858. - DOI - PubMed
-
- Cowling BJ, Park M, Fang VJ, Wu P, Leung GM, Wu JT. 2015. Preliminary epidemiological assessment of MERS-CoV outbreak in South Korea, May to June. Euro Surveill 20:pii=2113 http://dx.doi.org/10.28907/1560-7917.ES2015.25.21163. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

