Protein Neighbors and Proximity Proteomics
- PMID: 26355100
- PMCID: PMC4638030
- DOI: 10.1074/mcp.R115.052902
Protein Neighbors and Proximity Proteomics
Abstract
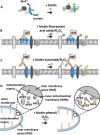
Within cells, proteins can co-assemble into functionally integrated and spatially restricted multicomponent complexes. Often, the affinities between individual proteins are relatively weak, and proteins within such clusters may interact only indirectly with many of their other protein neighbors. This makes proteomic characterization difficult using methods such as immunoprecipitation or cross-linking. Recently, several groups have described the use of enzyme-catalyzed proximity labeling reagents that covalently tag the neighbors of a targeted protein with a small molecule such as fluorescein or biotin. The modified proteins can then be isolated by standard pulldown methods and identified by mass spectrometry. Here we will describe the techniques as well as their similarities and differences. We discuss their applications both to study protein assemblies and to provide a new way for characterizing organelle proteomes. We stress the importance of proteomic quantitation and independent target validation in such experiments. Furthermore, we suggest that there are biophysical and cell-biological principles that dictate the appropriateness of enzyme-catalyzed proximity labeling methods to address particular biological questions of interest.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

Similar articles
-
Proteomic navigation using proximity-labeling.Methods. 2019 Jul 15;164-165:67-72. doi: 10.1016/j.ymeth.2019.03.028. Epub 2019 Apr 4. Methods. 2019. PMID: 30953756 Review.
-
Spatially resolved proteomic mapping in living cells with the engineered peroxidase APEX2.Nat Protoc. 2016 Mar;11(3):456-75. doi: 10.1038/nprot.2016.018. Epub 2016 Feb 11. Nat Protoc. 2016. PMID: 26866790 Free PMC article.
-
AirID, a novel proximity biotinylation enzyme, for analysis of protein-protein interactions.Elife. 2020 May 11;9:e54983. doi: 10.7554/eLife.54983. Elife. 2020. PMID: 32391793 Free PMC article.
-
APEX Peroxidase-Catalyzed Proximity Labeling and Multiplexed Quantitative Proteomics.Methods Mol Biol. 2019;2008:41-55. doi: 10.1007/978-1-4939-9537-0_4. Methods Mol Biol. 2019. PMID: 31124087
-
Proximity-dependent labeling methods for proteomic profiling in living cells.Wiley Interdiscip Rev Dev Biol. 2017 Jul;6(4):10.1002/wdev.272. doi: 10.1002/wdev.272. Epub 2017 Apr 7. Wiley Interdiscip Rev Dev Biol. 2017. PMID: 28387482 Free PMC article. Review.
Cited by
-
Proximity RNA Labeling by APEX-Seq Reveals the Organization of Translation Initiation Complexes and Repressive RNA Granules.Mol Cell. 2019 Aug 22;75(4):875-887.e5. doi: 10.1016/j.molcel.2019.07.030. Mol Cell. 2019. PMID: 31442426 Free PMC article.
-
Microenvironment mapping via Dexter energy transfer on immune cells.Science. 2020 Mar 6;367(6482):1091-1097. doi: 10.1126/science.aay4106. Science. 2020. PMID: 32139536 Free PMC article.
-
Generation of Peptides for Highly Efficient Proximity Utilizing Site-Specific Biotinylation in Cells.Life (Basel). 2022 Feb 16;12(2):300. doi: 10.3390/life12020300. Life (Basel). 2022. PMID: 35207587 Free PMC article.
-
New technologies to analyse protein function: an intrinsic disorder perspective.F1000Res. 2020 Feb 10;9:F1000 Faculty Rev-101. doi: 10.12688/f1000research.20867.1. eCollection 2020. F1000Res. 2020. PMID: 32089835 Free PMC article. Review.
-
In vivo Proximity Labeling of Nuclear and Nucleolar Proteins by a Stably Expressed, DNA Damage-Responsive NONO-APEX2 Fusion Protein.Front Mol Biosci. 2022 Jun 6;9:914873. doi: 10.3389/fmolb.2022.914873. eCollection 2022. Front Mol Biosci. 2022. PMID: 35733943 Free PMC article.
References
-
- Ellis R. J. (2001) Macromolecular crowding: an important but neglected aspect of the intracellular environment. Curr. Opin. Struct. Biol. 11, 114–119 - PubMed
-
- Zimmerman S. B., and Minton A. P. (1993) Macromolecular crowding: biochemical, biophysical, and physiological consequences. Annu. Rev. Biophys. Biomol. Struct. 22, 27–65 - PubMed
-
- Han J. D., Bertin N., Hao T., Goldberg D. S., Berriz G. F., Zhang L. V., Dupuy D., Walhout A. J., Cusick M. E., Roth F. P., and Vidal M. (2004) Evidence for dynamically organized modularity in the yeast protein-protein interaction network. Nature 430, 88–93 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

