Characteristic Changes in Cell Surface Glycosylation Accompany Intestinal Epithelial Cell (IEC) Differentiation: High Mannose Structures Dominate the Cell Surface Glycome of Undifferentiated Enterocytes
- PMID: 26355101
- PMCID: PMC4638035
- DOI: 10.1074/mcp.M115.053983
Characteristic Changes in Cell Surface Glycosylation Accompany Intestinal Epithelial Cell (IEC) Differentiation: High Mannose Structures Dominate the Cell Surface Glycome of Undifferentiated Enterocytes
Abstract
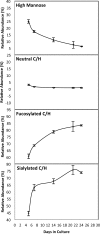
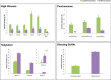
Changes in cell surface glycosylation occur during the development and differentiation of cells and have been widely correlated with the progression of several diseases. Because of their structural diversity and sensitivity to intra- and extracellular conditions, glycans are an indispensable tool for analyzing cellular transformations. Glycans present on the surface of intestinal epithelial cells (IEC) mediate interactions with billions of native microorganisms, which continuously populate the mammalian gut. A distinct feature of IECs is that they differentiate as they migrate upwards from the crypt base to the villus tip. In this study, nano-LC/ESI QTOF MS profiling was used to characterize the changes in glycosylation that correspond to Caco-2 cell differentiation. As Caco-2 cells differentiate to form a brush border membrane, a decrease in high mannose type glycans and a concurrent increase in fucosylated and sialylated complex/hybrid type glycans were observed. At day 21, when cells appear to be completely differentiated, remodeling of the cell surface glycome ceases. Differential expression of glycans during IEC maturation appears to play a key functional role in regulating the membrane-associated hydrolases and contributes to the mucosal surface innate defense mechanisms. Developing methodologies to rapidly identify changes in IEC surface glycans may lead to a rapid screening approach for a variety of disease states affecting the GI tract.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Moran A. P., Gupta A., and Joshi L. (2011) Sweet-talk: role of host glycosylation in bacterial pathogenesis of the gastrointestinal tract. Gut 60, 1412–1425 - PubMed
-
- Karlsson K. A. (1999) Bacterium-host protein-carbohydrate interactions and pathogenicity. Biochem. Soc. Trans. 27, 471–474 - PubMed
-
- van Kooyk Y., and Rabinovich G. A. (2008) Protein-glycan interactions in the control of innate and adaptive immune responses. Nat. Immunol. 9, 593–601 - PubMed
-
- Ohtsubo K., and Marth J. D. (2006) Glycosylation in cellular mechanisms of health and disease. Cell 126, 855–867 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

