Fstl1 Promotes Asthmatic Airway Remodeling by Inducing Oncostatin M
- PMID: 26355153
- PMCID: PMC4811198
- DOI: 10.4049/jimmunol.1501105
Fstl1 Promotes Asthmatic Airway Remodeling by Inducing Oncostatin M
Abstract
Chronic asthma is associated with airway remodeling and decline in lung function. In this article, we show that follistatin-like 1 (Fstl1), a mediator not previously associated with asthma, is highly expressed by macrophages in the lungs of humans with severe asthma. Chronic allergen-challenged Lys-Cre(tg) /Fstl1(Δ/Δ) mice in whom Fstl1 is inactivated in macrophages/myeloid cells had significantly reduced airway remodeling and reduced levels of oncostatin M (OSM), a cytokine previously not known to be regulated by Fstl1. The importance of the Fstl1 induction of OSM to airway remodeling was demonstrated in murine studies in which administration of Fstl1 induced airway remodeling and increased OSM, whereas administration of an anti-OSM Ab blocked the effect of Fstl1 on inducing airway remodeling, eosinophilic airway inflammation, and airway hyperresponsiveness, all cardinal features of asthma. Overall, these studies demonstrate that the Fstl1/OSM pathway may be a novel pathway to inhibit airway remodeling in severe human asthma.
Copyright © 2015 by The American Association of Immunologists, Inc.
Figures

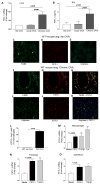
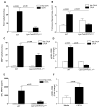
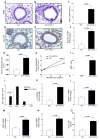
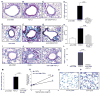
Similar articles
-
Autophagy plays a role in FSTL1-induced epithelial mesenchymal transition and airway remodeling in asthma.Am J Physiol Lung Cell Mol Physiol. 2017 Jul 1;313(1):L27-L40. doi: 10.1152/ajplung.00510.2016. Epub 2017 May 4. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28473327
-
FSTL1 aggravates OVA-induced inflammatory responses by activating the NLRP3/IL-1β signaling pathway in mice and macrophages.Inflamm Res. 2021 Jul;70(7):777-787. doi: 10.1007/s00011-021-01475-w. Epub 2021 Jun 2. Inflamm Res. 2021. PMID: 34076707
-
FSTL1 aggravates cigarette smoke-induced airway inflammation and airway remodeling by regulating autophagy.BMC Pulm Med. 2021 Jan 28;21(1):45. doi: 10.1186/s12890-021-01409-6. BMC Pulm Med. 2021. PMID: 33509151 Free PMC article.
-
Oncostatin M and interleukin-31: Cytokines, receptors, signal transduction and physiology.Cytokine Growth Factor Rev. 2015 Oct;26(5):545-58. doi: 10.1016/j.cytogfr.2015.07.006. Epub 2015 Jul 3. Cytokine Growth Factor Rev. 2015. PMID: 26198770 Review.
-
Bronchial epithelium as a target for innovative treatments in asthma.Pharmacol Ther. 2013 Dec;140(3):290-305. doi: 10.1016/j.pharmthera.2013.07.008. Epub 2013 Jul 21. Pharmacol Ther. 2013. PMID: 23880290 Review.
Cited by
-
Coordination of Immune-Stroma Crosstalk by IL-6 Family Cytokines.Front Immunol. 2019 May 15;10:1093. doi: 10.3389/fimmu.2019.01093. eCollection 2019. Front Immunol. 2019. PMID: 31156640 Free PMC article. Review.
-
Neutrophils are a major source of the epithelial barrier disrupting cytokine oncostatin M in patients with mucosal airways disease.J Allergy Clin Immunol. 2017 Jun;139(6):1966-1978.e9. doi: 10.1016/j.jaci.2016.10.039. Epub 2016 Dec 18. J Allergy Clin Immunol. 2017. PMID: 27993536 Free PMC article.
-
The barrier hypothesis and Oncostatin M: Restoration of epithelial barrier function as a novel therapeutic strategy for the treatment of type 2 inflammatory disease.Tissue Barriers. 2017 Jul 3;5(3):e1341367. doi: 10.1080/21688370.2017.1341367. Epub 2017 Jun 13. Tissue Barriers. 2017. PMID: 28665760 Free PMC article. Review.
-
Case-Control Study: Evaluating the Role and Therapeutic Potential of FSTL1 in Type 2 Inflammation of Chronic Rhinosinusitis.J Inflamm Res. 2025 Feb 20;18:2545-2556. doi: 10.2147/JIR.S507059. eCollection 2025. J Inflamm Res. 2025. PMID: 39995823 Free PMC article.
-
Follistatin-like 1 and Biomarkers of Neutrophil Activation Are Associated with Poor Short-Term Outcome after Lung Transplantation on VA-ECMO.Biology (Basel). 2022 Oct 8;11(10):1475. doi: 10.3390/biology11101475. Biology (Basel). 2022. PMID: 36290379 Free PMC article.
References
-
- Shibanuma M, Mashimo J, Mita A, Kuroki T, Nose K. Cloning from a mouse osteoblastic cell line of a set of transforming-growth-factor-beta 1-regulated genes, one of which seems to encode a follistatin-related polypeptide. Eur J Biochem. 1993;217:13–19. - PubMed
-
- Tanaka M, Ozaki S, Osakada F, Mori K, Okubo M, Nakao K. Cloning of follistatin-related protein as a novel autoantigen in systemic rheumatic diseases. Int Immunol. 1998;10:1305–1314. - PubMed
-
- Chaly Y, Hostager B, Smith S, Hirsch R. Follistatin-like protein 1 and its role in inflammation and inflammatory diseases. Immunol Res. 2014;59:266–272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

