Frenemies: Signaling and Nutritional Integration in Pathogen-Microbiota-Host Interactions
- PMID: 26355214
- PMCID: PMC4567707
- DOI: 10.1016/j.chom.2015.08.007
Frenemies: Signaling and Nutritional Integration in Pathogen-Microbiota-Host Interactions
Abstract
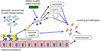
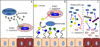
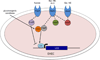
The mammalian gastrointestinal (GI) microbiota is highly adapted to thrive in the GI environment and performs key functions related to host nutrition, physiology, development, immunity, and behavior. Successful host-bacterial associations require chemical signaling and optimal nutrient utilization and exchange. However, this important balance can be severely disrupted by environmental stimuli, with one of the most common insults upon the microbiota being infectious diseases. Although the microbiota acts as a barrier toward enteric pathogens, many enteric pathogens exploit signals and nutrients derived from both the microbiota and host to regulate their virulence programs. Here we review several signaling and nutrient recognition systems employed by GI pathogens to regulate growth and virulence. We discuss how shifts in the microbiota composition change host susceptibility to infection and how dietary changes or manipulation of the microbiota could potentially prevent and/or ameliorate GI infections.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
References
-
- Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K, Koga Y, Sudo N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1288–G1295. - PubMed
-
- Bai J, McAteer SP, Paxton E, Mahajan A, Gally DL, Tree JJ. Screening of an E. coli O157:H7 Bacterial Artificial Chromosome Library by Comparative Genomic Hybridization to Identify Genomic Regions Contributing to Growth in Bovine Gastrointestinal Mucus and Epithelial Cell Colonization. Front Microbiol. 2011;2:168. - PMC - PubMed
-
- Bertin Y, Girardeau JP, Chaucheyras-Durand F, Lyan B, Pujos-Guillot E, Harel J, Martin C. Enterohaemorrhagic Escherichia coli gains a competitive advantage by using ethanolamine as a nitrogen source in the bovine intestinal content. Environ Microbiol. 2011;13:365–377. - PubMed
-
- Bohnhoff M, Drake BL, Miller CP. Effect of streptomycin on susceptibility of intestinal tract to experimental Salmonella infection. Proc Soc Exp Biol Med. 1954;86:132–137. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

