Current Knowledge on Hepatitis E
- PMID: 26355220
- PMCID: PMC4548356
- DOI: 10.14218/JCTH.2015.00009
Current Knowledge on Hepatitis E
Abstract
Although only a single serotype of hepatitis E virus (HEV), the causative agent of hepatitis E, has been identified, there is great genetic variation among the different HEV isolates reported. There are at least four major recognized genotypes of HEV: genotypes 1 and 2 are mainly restricted to humans and linked to epidemic outbreaks in nonindustrialized countries, whereas genotypes 3 and 4 are zoonotic in both developing and industrialized countries. Besides human strains, genotype 3 and 4 strains of HEV have been genetically characterized from swine, sika deer, mongooses, sheep, and rabbits. Currently, there are approximately 11,000 human and animal sequences of HEV available at the International Nucleotide Sequence Database Collaboration. HEV is the major cause of waterborne outbreaks of hepatitis in areas of poor sanitation. Additionally, it is responsible for sporadic cases of viral hepatitis in not only endemic but industrialized countries as well. Transmission of HEV occurs predominantly by the fecal-oral route, although parenteral and perinatal routes have been reported. HEV infection develops in most individuals as a self-limiting, acute, icteric hepatitis; with mortality rates around 1%. However, some affected individuals will develop fulminant hepatic failure, a serious condition that is frequently fatal without a liver transplant. This complication is particularly common when the infection occurs in pregnant women, where mortality rates rise dramatically to up to 25%. Among the preventive measures available to avoid HEV infection, two separate subunit vaccines containing recombinant truncated capsid proteins of HEV have been shown to be highly effective in the prevention of disease. One of them, HEV 239, was approved in China, and its commercialization by Innovax began in November 2012 under the name Hecolin(®).
Keywords: HEV; Hepatitis E; Hepatitis E virus; Zoonosis.
Conflict of interest statement
Figures
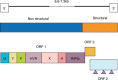
 , glicosilation site.
, glicosilation site.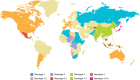
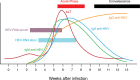
References
-
- Pérez-Gracia MT, Mateos-Lindemann ML. Hepatitis E. Current perspectives. Med Clin (Barc) 2012;139:404–411. 10.1016/j.medcli.2012.02.013. - DOI - PubMed
-
- Pérez-Gracia MT, Mateos Lindemann ML, Montalvo Villalba MC. Hepatitis E: current status. Rev Med Virol. 2013;23:384–398. 10.1002/rmv.1759. - DOI - PubMed
-
- Balayan MS, Andjaparidze AG, Savinskaya SS, Ketiladze ES, Braginsky DM, Savinov AP, et al. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology. 1983;20:23–31. - PubMed
-
- Reyes GR, Purdy MA, Kim JP, Luk KC, Young LM, Fry KE, et al. Isolation of a cDNA from the virus responsible for enterically transmitted non-A, non-B hepatitis. Science. 1990;247:1335–1339. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
