Inactivated Eyedrop Influenza Vaccine Adjuvanted with Poly(I:C) Is Safe and Effective for Inducing Protective Systemic and Mucosal Immunity
- PMID: 26355295
- PMCID: PMC4565664
- DOI: 10.1371/journal.pone.0137608
Inactivated Eyedrop Influenza Vaccine Adjuvanted with Poly(I:C) Is Safe and Effective for Inducing Protective Systemic and Mucosal Immunity
Abstract
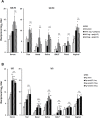
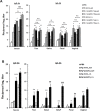
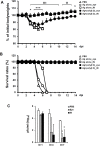
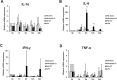
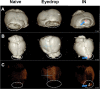
The eye route has been evaluated as an efficient vaccine delivery routes. However, in order to induce sufficient antibody production with inactivated vaccine, testing of the safety and efficacy of the use of inactivated antigen plus adjuvant is needed. Here, we assessed various types of adjuvants in eyedrop as an anti-influenza serum and mucosal Ab production-enhancer in BALB/c mice. Among the adjuvants, poly (I:C) showed as much enhancement in antigen-specific serum IgG and mucosal IgA antibody production as cholera toxin (CT) after vaccinations with trivalent hemagglutinin-subunits or split H1N1 vaccine antigen in mice. Vaccination with split H1N1 eyedrop vaccine antigen plus poly(I:C) showed a similar or slightly lower efficacy in inducing antibody production than intranasal vaccination; the eyedrop vaccine-induced immunity was enough to protect mice from lethal homologous influenza A/California/04/09 (H1N1) virus challenge. Additionally, ocular inoculation with poly(I:C) plus vaccine antigen generated no signs of inflammation within 24 hours: no increases in the mRNA expression levels of inflammatory cytokines nor in the infiltration of mononuclear cells to administration sites. In contrast, CT administration induced increased expression of IL-6 cytokine mRNA and mononuclear cell infiltration in the conjunctiva within 24 hours of vaccination. Moreover, inoculated visualizing materials by eyedrop did not contaminate the surface of the olfactory bulb in mice; meanwhile, intranasally administered materials defiled the surface of the brain. On the basis of these findings, we propose that the use of eyedrop inactivated influenza vaccine plus poly(I:C) is a safe and effective mucosal vaccine strategy for inducing protective anti-influenza immunity.
Conflict of interest statement
Figures


Similar articles
-
Bacterium-like particles supplemented with inactivated influenza antigen induce cross-protective influenza-specific antibody responses through intranasal administration.Vaccine. 2012 Jul 6;30(32):4884-91. doi: 10.1016/j.vaccine.2012.04.032. Epub 2012 Apr 23. Vaccine. 2012. PMID: 22537989
-
Eye mucosa: an efficient vaccine delivery route for inducing protective immunity.J Immunol. 2010 Sep 15;185(6):3610-9. doi: 10.4049/jimmunol.1000680. Epub 2010 Aug 13. J Immunol. 2010. PMID: 20709955
-
Intranasal administration of a flagellin-adjuvanted inactivated influenza vaccine enhances mucosal immune responses to protect mice against lethal infection.Vaccine. 2012 Jan 5;30(2):466-74. doi: 10.1016/j.vaccine.2011.10.058. Epub 2011 Oct 31. Vaccine. 2012. PMID: 22051136
-
Studies on the usefulness of intranasal inactivated influenza vaccines.Vaccine. 2010 Aug 31;28(38):6393-7. doi: 10.1016/j.vaccine.2010.05.019. Epub 2010 May 20. Vaccine. 2010. PMID: 20493820 Review.
-
A proposal for safety standards for human use of cholera toxin (or Escherichia coli heat-labile enterotoxin) derivatives as an adjuvant of nasal inactivated influenza vaccine.Jpn J Infect Dis. 2000 Jun;53(3):98-106. Jpn J Infect Dis. 2000. PMID: 10957706 Review.
Cited by
-
Mucosal Vaccination Primes NK Cell-Dependent Development of CD8+ T Cells Against Pulmonary Brucella Infection.Front Immunol. 2021 Jul 7;12:697953. doi: 10.3389/fimmu.2021.697953. eCollection 2021. Front Immunol. 2021. PMID: 34305935 Free PMC article.
-
Evaluation of the Protective Efficacy of Poly I:C as an Adjuvant for H9N2 Subtype Avian Influenza Inactivated Vaccine and Its Mechanism of Action in Ducks.PLoS One. 2017 Jan 30;12(1):e0170681. doi: 10.1371/journal.pone.0170681. eCollection 2017. PLoS One. 2017. PMID: 28135294 Free PMC article.
-
Inducing Mucosal IgA: A Challenge for Vaccine Adjuvants and Delivery Systems.J Immunol. 2017 Jul 1;199(1):9-16. doi: 10.4049/jimmunol.1601775. J Immunol. 2017. PMID: 28630108 Free PMC article. Review.
-
Immunogenicity of Exosomes from Dendritic Cells Stimulated with Toxoplasma gondii Lysates in Ocularly Immunized Mice.Korean J Parasitol. 2020 Apr;58(2):185-189. doi: 10.3347/kjp.2020.58.2.185. Epub 2020 Apr 30. Korean J Parasitol. 2020. PMID: 32418388 Free PMC article.
-
Mucosal and Systemic Immune Responses to Influenza H7N9 Antigen HA1-2 Co-Delivered Intranasally with Flagellin or Polyethyleneimine in Mice and Chickens.Front Immunol. 2017 Apr 5;8:326. doi: 10.3389/fimmu.2017.00326. eCollection 2017. Front Immunol. 2017. PMID: 28424686 Free PMC article.
References
-
- Tamura S, Hasegawa H, Kurata T. Estimation of the effective doses of nasal-inactivated influenza vaccine in humans from mouse-model experiments. Jpn J Infect Dis. 2010;63(1):8–15. Epub 2010/01/23. . - PubMed
-
- Centers for Disease Control and Prevention (CDC). Influenza vaccine: live, intranasal 2012–2013 Atlanta: CDC; 2012. [updated July 2, 2012]. Available: http://www.cdc.gov/vaccines/hcp/vis/vis-statements/flulive.pdf.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

