Effects of Menthol on Nicotine Pharmacokinetic, Pharmacology and Dependence in Mice
- PMID: 26355604
- PMCID: PMC4565647
- DOI: 10.1371/journal.pone.0137070
Effects of Menthol on Nicotine Pharmacokinetic, Pharmacology and Dependence in Mice
Abstract
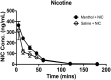
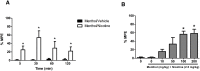
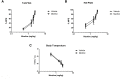
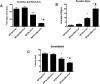
Although menthol, a common flavoring additive to cigarettes, has been found to impact the addictive properties of nicotine cigarettes in smokers little is known about its pharmacological and molecular actions in the brain. Studies were undertaken to examine whether the systemic administration of menthol would modulate nicotine pharmacokinetics, acute pharmacological effects (antinociception and hypothermia) and withdrawal in male ICR mice. In addition, we examined changes in the brain levels of nicotinic receptors of rodents exposed to nicotine and menthol. Administration of i.p. menthol significantly decreased nicotine's clearance (2-fold decrease) and increased its AUC compared to i.p. vehicle treatment. In addition, menthol pretreatment prolonged the duration of nicotine-induced antinociception and hypothermia (2.5 mg/kg, s.c.) for periods up to 180 min post-nicotine administration. Repeated administration of menthol with nicotine increased the intensity of mecamylamine-precipitated withdrawal signs in mice exposed chronically to nicotine. The potentiation of withdrawal intensity by menthol was accompanied by a significant increase in nicotine plasma levels in these mice. Western blot analyses of α4 and β2 nAChR subunit expression suggests that chronic menthol impacts the levels and distribution of these nicotinic subunits in various brain regions. In particular, co-administration of menthol and nicotine appears to promote significant increase in β2 and α4 nAChR subunit expression in the hippocampus, prefrontal cortex and striatum of mice. Surprisingly, chronic injections of menthol alone to mice caused an upregulation of β2 and α4 nAChR subunit levels in these brain regions. Because the addition of menthol to tobacco products has been suggested to augment their addictive potential, the current findings reveal several new pharmacological molecular adaptations that may contribute to its unique addictive profile.
Conflict of interest statement
Figures

References
-
- Benowitz NL, Herrera B, Jacob P III. Mentholated cigarette smoking inhibits nicotine metabolism. J Pharmacol Exp Ther. 2004; 310(3), 1208–1215. - PubMed
-
- Williams JM, Gandhi KK, Steinberg ML, Foulds J, Ziedonis DM, Benowitz NL. Higher nicotine and carbon monoxide levels in menthol cigarette smokers with and without schizophrenia. Nicotine Tob Res. 2007. August; 9(8):873–81. - PubMed
-
- Okuyemi KS, Faseru B, Sanderson Cox L, Bronars CA, Ahluwalia JS. Relationship between menthol cigarettes and smoking cessation among African American light smokers. Addiction. 2007;102(12):1979–86. - PubMed
-
- Pletchr MJ, Hulley BJ, Houston T, Kiefe CI, Benowitz N, Sidney S. Menthol cigarettes, smoking cessation, atherosclerosis, and pulmonary function: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Arch Intern Med. 2006. September 25;166(17):1915–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

