Matrix Metalloproteinase-2 (MMP-2) Gene Deletion Enhances MMP-9 Activity, Impairs PARP-1 Degradation, and Exacerbates Hepatic Ischemia and Reperfusion Injury in Mice
- PMID: 26355684
- PMCID: PMC4565667
- DOI: 10.1371/journal.pone.0137642
Matrix Metalloproteinase-2 (MMP-2) Gene Deletion Enhances MMP-9 Activity, Impairs PARP-1 Degradation, and Exacerbates Hepatic Ischemia and Reperfusion Injury in Mice
Abstract
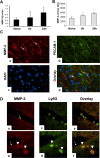
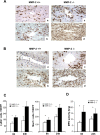
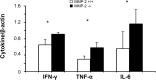
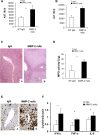
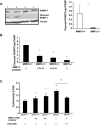
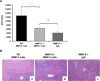
Hepatic ischemia and reperfusion injury (IRI) is an inflammatory condition and a significant cause of morbidity and mortality after surgery. Matrix metalloproteinases (MMPs) have been widely implicated in the pathogenesis of inflammatory diseases. Among the different MMPs, gelatinases (MMP-2 and MMP-9) are within the most prominent MMPs detected during liver IRI. While the role of MMP-9 in liver damage has been fairly documented, direct evidence of the role for MMP-2 activity in hepatic IRI remains to be established. Due to the lack of suitable inhibitors to target individual MMPs in vivo, gene manipulation is as an essential tool to assess MMP direct contribution to liver injury. Hence, we used MMP-2-/- deficient mice and MMP-2+/+ wild-type littermates to examine the function of MMP-2 activity in hepatic IRI. MMP-2 expression was detected along the sinusoids of wild-type livers before and after surgery and in a small population of leukocytes post-IRI. Compared to MMP-2+/+ mice, MMP-2 null (MMP-2-/-) mice showed exacerbated liver damage at 6, 24, and 48 hours post-reperfusion, which was fatal in some cases. MMP-2 deficiency resulted in upregulation of MMP-9 activity, spontaneous leukocyte infiltration in naïve livers, and amplified MMP-9-dependent transmigration of leukocytes in vitro and after hepatic IRI. Moreover, complete loss of MMP-2 activity impaired the degradation of poly (ADP-ribose) polymerase (PARP-1) in extensively damaged livers post-reperfusion. However, the administration of a PARP-1 inhibitor to MMP-2 null mice restored liver preservation to almost comparable levels of MMP-2+/+ mice post-IRI. Deficient PARP-1 degradation in MMP-2-null sinusoidal endothelial cells correlated with their increased cytotoxicity, evaluated by the measurement of LDH efflux in the medium. In conclusion, our results show for the first time that MMP-2 gene deletion exacerbates liver IRI. Moreover, they offer new insights into the MMP-2 modulation of inflammatory responses, which could be relevant for the design of new pharmacological MMP-targeted agents to treat hepatic IRI.
Conflict of interest statement
Figures



Similar articles
-
MMP-9 deficiency shelters endothelial PECAM-1 expression and enhances regeneration of steatotic livers after ischemia and reperfusion injury.J Hepatol. 2014 May;60(5):1032-9. doi: 10.1016/j.jhep.2013.12.022. Epub 2014 Jan 8. J Hepatol. 2014. PMID: 24412604 Free PMC article.
-
Leukocyte transmigration across endothelial and extracellular matrix protein barriers in liver ischemia/reperfusion injury.Curr Opin Organ Transplant. 2011 Feb;16(1):34-40. doi: 10.1097/MOT.0b013e328342542e. Curr Opin Organ Transplant. 2011. PMID: 21150609 Free PMC article. Review.
-
Metalloproteinase-9 deficiency protects against hepatic ischemia/reperfusion injury.Hepatology. 2008 Jan;47(1):186-98. doi: 10.1002/hep.21922. Hepatology. 2008. PMID: 17880014
-
Fibronectin-α4β1 interactions in hepatic cold ischemia and reperfusion injury: regulation of MMP-9 and MT1-MMP via the p38 MAPK pathway.Am J Transplant. 2012 Oct;12(10):2689-99. doi: 10.1111/j.1600-6143.2012.04161.x. Epub 2012 Jul 19. Am J Transplant. 2012. PMID: 22812390 Free PMC article.
-
The role of matrix metalloproteinase inhibitors in ischemia-reperfusion injury in the liver.Curr Pharm Des. 2006;12(23):2923-34. doi: 10.2174/138161206777947560. Curr Pharm Des. 2006. PMID: 16918422 Review.
Cited by
-
E-cigarette-Induced Pulmonary Inflammation and Dysregulated Repair are Mediated by nAChR α7 Receptor: Role of nAChR α7 in ACE2 Covid-19 receptor regulation.Res Sq [Preprint]. 2020 May 18:rs.2.23829. doi: 10.21203/rs.2.23829/v2. Res Sq. 2020. Update in: Respir Res. 2020 Jun 18;21(1):154. doi: 10.1186/s12931-020-01396-y. PMID: 32702718 Free PMC article. Updated. Preprint.
-
Nobiletin ameliorates hepatic ischemia and reperfusion injury through the activation of SIRT-1/FOXO3a-mediated autophagy and mitochondrial biogenesis.Exp Mol Med. 2019 Apr 26;51(4):1-16. doi: 10.1038/s12276-019-0245-z. Exp Mol Med. 2019. PMID: 31028246 Free PMC article.
-
Morroniside protects against cerebral ischemia/reperfusion injury by inhibiting neuron apoptosis and MMP2/9 expression.Exp Ther Med. 2018 Sep;16(3):2229-2234. doi: 10.3892/etm.2018.6457. Epub 2018 Jul 17. Exp Ther Med. 2018. PMID: 30186462 Free PMC article.
-
E-cigarette-induced pulmonary inflammation and dysregulated repair are mediated by nAChR α7 receptor: role of nAChR α7 in SARS-CoV-2 Covid-19 ACE2 receptor regulation.Respir Res. 2020 Jun 18;21(1):154. doi: 10.1186/s12931-020-01396-y. Respir Res. 2020. PMID: 32552811 Free PMC article.
-
Overproduction of Tenascin-C Driven by Lipid Accumulation in the Liver Aggravates Hepatic Ischemia/Reperfusion Injury in Steatotic Mice.Liver Transpl. 2019 Feb;25(2):288-301. doi: 10.1002/lt.25365. Liver Transpl. 2019. PMID: 30358115 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

