Pharmacokinetic and nephroprotective benefits of using Schisandra chinensis extracts in a cyclosporine A-based immune-suppressive regime
- PMID: 26355803
- PMCID: PMC4560515
- DOI: 10.2147/DDDT.S89876
Pharmacokinetic and nephroprotective benefits of using Schisandra chinensis extracts in a cyclosporine A-based immune-suppressive regime
Abstract
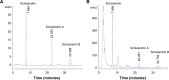
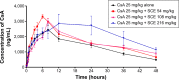
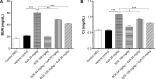
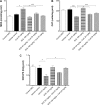
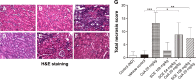
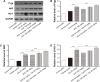
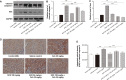
Cyclosporine A (CsA) is a powerful immunosuppressive drug. However, nephrotoxicity resulting from its long-term usage has hampered its prolonged therapeutic usage. Schisandra chinensis extracts (SCE) have previously been used in traditional Chinese medicine and more recently coadministered with Western medicine for the treatment of CsA-induced side effects in the People's Republic of China. This study aimed to investigate the possible effects of SCE on the pharmacokinetics of CsA in rats and elucidate the potential mechanisms by which it hinders the development of CsA-induced nephrotoxicity. A liquid chromatography/tandem mass spectrometry method was developed and validated for determining the effect of SCE on the pharmacokinetics of CsA. Male Sprague Dawley rats, which were administered with CsA (25 mg/kg/d) alone or in combination with SCE (54 mg/kg/d and 108 mg/kg/d) for 28 days, were used to evaluate the nephroprotective effects of SCE. Our study showed that SCE increased the mean blood concentration of CsA. Furthermore, we found that the concomitant administration of SCE alongside CsA prevented the disruption of catalase activity and reduction in creatinine, urea, renal malondialdehyde, and glutathione peroxidase levels that would have otherwise occurred in the absence of SCE administration. SCE treatment markedly suppressed the expression of 4-hydroxynonenal, Bcl-2-associated X protein, cleaved caspase 3, and autophagy-related protein LC3 A/B. On the other hand, the expression of heme oxygenase-1, nuclear factor erythroid 2-related factor 2 (Nrf2), and P-glycoprotein was enhanced by the very same addition of SCE. SCE was also able to increase the systemic exposure of CsA in rats. The renoprotective effects of SCE were thought to be mediated by its antiapoptotic and antioxidant abilities, which caused the attenuation of CsA-induced autophagic cell death. All in all, these findings suggest the prospective use of SCE as an effective adjunct in a CsA-based immunosuppressive regimen.
Keywords: Schisandra chinensis extracts; apoptosis; autophagy; cyclosporine A; nephroprotective; oxidative stress; pharmacokinetics.
Figures




Similar articles
-
Improvement of Cisplatin-induced renal dysfunction by Schisandra chinensis stems via anti-inflammation and anti-apoptosis effects.J Ethnopharmacol. 2018 May 10;217:228-237. doi: 10.1016/j.jep.2018.01.033. Epub 2018 Feb 6. J Ethnopharmacol. 2018. PMID: 29421595
-
Protective effect of schisandrin B against cyclosporine A-induced nephrotoxicity in vitro and in vivo.Am J Chin Med. 2012;40(3):551-66. doi: 10.1142/S0192415X12500425. Am J Chin Med. 2012. PMID: 22745070
-
Schisandra chinensis extract decreases chloroacetaldehyde production in rats and attenuates cyclophosphamide toxicity in liver, kidney and brain.J Ethnopharmacol. 2018 Jan 10;210:223-231. doi: 10.1016/j.jep.2017.08.020. Epub 2017 Aug 15. J Ethnopharmacol. 2018. PMID: 28821392
-
Mechanism of cyclosporine A nephrotoxicity: Oxidative stress, autophagy, and signalings.Food Chem Toxicol. 2018 Aug;118:889-907. doi: 10.1016/j.fct.2018.06.054. Epub 2018 Jun 28. Food Chem Toxicol. 2018. PMID: 29960018 Review.
-
Prevention of nephrotoxicity induced by cyclosporine-A: role of antioxidants.J Cell Biochem. 2015 Mar;116(3):364-9. doi: 10.1002/jcb.25022. J Cell Biochem. 2015. PMID: 25418335 Review.
Cited by
-
Natural Reno-Protective Agents against Cyclosporine A-Induced Nephrotoxicity: An Overview.Molecules. 2022 Nov 11;27(22):7771. doi: 10.3390/molecules27227771. Molecules. 2022. PMID: 36431872 Free PMC article. Review.
-
New Potential Pharmacological Functions of Chinese Herbal Medicines via Regulation of Autophagy.Molecules. 2016 Mar 17;21(3):359. doi: 10.3390/molecules21030359. Molecules. 2016. PMID: 26999089 Free PMC article. Review.
-
A Comprehensive Review of the Main Lignan Components of Schisandra chinensis (North Wu Wei Zi) and Schisandra sphenanthera (South Wu Wei Zi) and the Lignan-Induced Drug-Drug Interactions Based on the Inhibition of Cytochrome P450 and P-Glycoprotein Activities.Front Pharmacol. 2022 Mar 11;13:816036. doi: 10.3389/fphar.2022.816036. eCollection 2022. Front Pharmacol. 2022. PMID: 35359848 Free PMC article. Review.
-
Schisandrin B elicits the Keap1-Nrf2 defense system via carbene reactive metabolite which is less harmful to mice liver.Drug Des Devel Ther. 2018 Nov 23;12:4033-4046. doi: 10.2147/DDDT.S176561. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 30568426 Free PMC article.
-
Nephroprotective activity of natural products against chemical toxicants: The role of Nrf2/ARE signaling pathway.Food Sci Nutr. 2021 May 1;9(6):3362-3384. doi: 10.1002/fsn3.2320. eCollection 2021 Jun. Food Sci Nutr. 2021. PMID: 34136201 Free PMC article. Review.
References
-
- Grinyo JM, Cruzado JM. Cyclosporine nephrotoxicity. Transplant Proc. 2004;36(2):240S–242S. - PubMed
-
- Mariee AD, Abd-Ellah MF. Protective effect of docosahexaenoic acid against cyclosporine A-induced nephrotoxicity in rats: a possible mechanism of action. Ren Fail. 2011;33(1):66–71. - PubMed
-
- Tavares P, Fontes Ribeiro CA, Teixeira F. Cyclosporin effect on noradrenaline release from the sympathetic nervous endings of rat aorta. Pharmacol Res. 2003;47(1):27–33. - PubMed
-
- Capasso G, Di Gennaro CI, Della Ragione F, et al. In vivo effect of the natural antioxidant hydroxytyrosol on cyclosporine nephrotoxicity in rats. Nephrol Dial Transplant. 2008;23(4):1186–1195. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

