Impact of Subunit Composition on the Uptake of α-Crystallin by Lens and Retina
- PMID: 26355842
- PMCID: PMC4565700
- DOI: 10.1371/journal.pone.0137659
Impact of Subunit Composition on the Uptake of α-Crystallin by Lens and Retina
Abstract
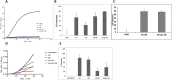
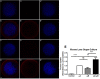
Misfolded protein aggregation, including cataract, cause a significant amount of blindness worldwide. α-Crystallin is reported to bind misfolded proteins and prevent their aggregation. We hypothesize that supplementing retina and lens with α-crystallin may help to delay disease onset. The purpose of this study was to determine if αB-crystallin subunits containing a cell penetration peptide (gC-tagged αB-crystallin) facilitate the uptake of wild type αA-crystallin (WT-αA) in lens and retina. Recombinant human αB-crystallin was modified by the addition of a novel cell penetration peptide derived from the gC gene product of herpes simplex virus (gC-αB). Recombinant gC-αB and wild-type αA-crystallin (WT-αA) were purified from E. coli over-expression cultures. After Alexa-labeling of WT-αA, these proteins were mixed at ratios of 1:2, 1:5 and 1:10, respectively, and incubated at 37°C for 4 hours to allow for subunit exchange. Mixed oligomers were subsequently incubated with tissue culture cells or mouse organ cultures. Similarly, crystallin mixtures were injected into the vitreous of rat eyes. At various times after exposure, tissues were harvested and analyzed for protein uptake by confocal microscopy or flow cytometry. Chaperone-like activity assays were performed on α-crystallins ratios showing optimal uptake using chemically-induced or heat induced substrate aggregation assays. As determined by flow cytometry, a ratio of 1:5 for gC-αB to WT-αA was found to be optimal for uptake into retinal pigmented epithelial cells (ARPE-19). Chaperone-like activity assays demonstrated that hetero-oligomeric complex of gC-αB to WT-αA (in 1:5 ratio) retained protein aggregation protection. We observed a significant increase in protein uptake when optimized (gC-αB to WT-αA (1:5 ratio)) hetero-oligomers were used in mouse lens and retinal organ cultures. Increased levels of α-crystallin were found in lens and retina following intravitreal injection of homo- and hetero-oligomers in rats.
Conflict of interest statement
Figures



Similar articles
-
Oligomerization with wt αA- and αB-crystallins reduces proteasome-mediated degradation of C-terminally truncated αA-crystallin.Invest Ophthalmol Vis Sci. 2012 May 4;53(6):2541-50. doi: 10.1167/iovs.11-9147. Invest Ophthalmol Vis Sci. 2012. PMID: 22427585 Free PMC article.
-
Cell penetration peptides for enhanced entry of αB-crystallin into lens cells.Invest Ophthalmol Vis Sci. 2013 Jan 2;54(1):2-8. doi: 10.1167/iovs.12-10947. Invest Ophthalmol Vis Sci. 2013. PMID: 23150610 Free PMC article.
-
Cataract mutation P20S of alphaB-crystallin impairs chaperone activity of alphaA-crystallin and induces apoptosis of human lens epithelial cells.Biochim Biophys Acta. 2008 May;1782(5):303-9. doi: 10.1016/j.bbadis.2008.01.011. Epub 2008 Feb 20. Biochim Biophys Acta. 2008. PMID: 18343237
-
Alpha crystallins in the retinal pigment epithelium and implications for the pathogenesis and treatment of age-related macular degeneration.Biochim Biophys Acta. 2016 Jan;1860(1 Pt B):258-68. doi: 10.1016/j.bbagen.2015.05.016. Epub 2015 May 27. Biochim Biophys Acta. 2016. PMID: 26026469 Free PMC article. Review.
-
Regulation of αA- and αB-crystallins via phosphorylation in cellular homeostasis.Cell Mol Life Sci. 2015 Nov;72(21):4127-37. doi: 10.1007/s00018-015-1996-x. Epub 2015 Jul 26. Cell Mol Life Sci. 2015. PMID: 26210153 Free PMC article. Review.
Cited by
-
Localization of αA-Crystallin in Rat Retinal Müller Glial Cells and Photoreceptors.Microsc Microanal. 2018 Oct;24(5):545-552. doi: 10.1017/S1431927618015118. Epub 2018 Sep 26. Microsc Microanal. 2018. PMID: 30253817 Free PMC article.
-
Changes in function but not oligomeric size are associated with αB-crystallin lysine substitution.Biochem Biophys Rep. 2018 Mar 30;14:1-6. doi: 10.1016/j.bbrep.2018.03.001. eCollection 2018 Jul. Biochem Biophys Rep. 2018. PMID: 29872727 Free PMC article.
-
The Protective Effects of αB-Crystallin on Ischemia-Reperfusion Injury in the Rat Retina.J Ophthalmol. 2017;2017:7205408. doi: 10.1155/2017/7205408. Epub 2017 Oct 2. J Ophthalmol. 2017. PMID: 29098085 Free PMC article.
References
-
- Bloemendal H. The Lens Proteins Molecular and Cellular Biology of the Eye Lens. New York: John Wiley & Sons; 1981. p. 1–14.
-
- Gopalakrishnan S, Takemoto L. An assay for intermolecular exchange of alpha crystallin. Investigative Ophthalmology & Visual Science. 1992;33:2936–41. - PubMed
-
- Bova MP, Ding LL, Horwitz J, Fung BK. Subunit exchange of alphaA-crystallin. Journal Of Biological Chemistry. 1997;272(47):29511–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

