Disruption in dopaminergic innervation during photoreceptor degeneration
- PMID: 26356010
- PMCID: PMC4747828
- DOI: 10.1002/cne.23899
Disruption in dopaminergic innervation during photoreceptor degeneration
Abstract
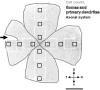
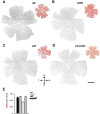
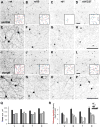
Dopaminergic amacrine cells (DACs) release dopamine in response to light-driven synaptic inputs, and are critical to retinal light adaptation. Retinal degeneration (RD) compromises the light responsiveness of the retina and, subsequently, dopamine metabolism is impaired. As RD progresses, retinal neurons exhibit aberrant activity, driven by AII amacrine cells, a primary target of the retinal dopaminergic network. Surprisingly, DACs are an exception to this physiological change; DACs exhibit rhythmic activity in healthy retina, but do not burst in RD. The underlying mechanism of this divergent behavior is not known. It is also unclear whether RD leads to structural changes in DACs, impairing functional regulation of AII amacrine cells. Here we examine the anatomical details of DACs in three mouse models of human RD to determine how changes to the dopaminergic network may underlie physiological changes in RD. By using rd10, rd1, and rd1/C57 mice we were able to dissect the impacts of genetic background and the degenerative process on DAC structure in RD retina. We found that DACs density, soma size, and primary dendrite length are all significantly reduced. Using a novel adeno-associated virus-mediated technique to label AII amacrine cells in mouse retina, we observed diminished dopaminergic contacts to AII amacrine cells in RD mice. This was accompanied by changes to the components responsible for dopamine synthesis and release. Together, these data suggest that structural alterations of the retinal dopaminergic network underlie physiological changes during RD.
Keywords: RRID:AB_2315595; RRID:AB_572268; RRID:Jax:00002; RRID:Jax:000659; RRID:Jax:000664; RRID:Jax:004297; RRID:nif-0000-30467; RRID:nlx_157306; RRID:rid_000042; amacrine cell; dopamine; gap junctions; retinal degeneration.
© 2015 Wiley Periodicals, Inc.
Conflict of interest statement
All authors declare they have no conflict of interest.
Figures





References
-
- Atkinson CL, Feng J, Zhang DQ. Functional integrity and modification of retinal dopaminergic neurons in the rd1 mutant mouse: roles of melanopsin and GABA. J Neurophysiol. 2013;109(6):1589–1599. - PubMed
-
- Chang GQ, Gaitan A, Hao Y, Wong F. Correlation of DNA fragmentation and chromatin condensation in apoptotic nuclei of the Ser 6 mouse retina. Microsc Res Tech. 1997;36(2):123–129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

