Dual loss of the SWI/SNF complex ATPases SMARCA4/BRG1 and SMARCA2/BRM is highly sensitive and specific for small cell carcinoma of the ovary, hypercalcaemic type
- PMID: 26356327
- PMCID: PMC4832362
- DOI: 10.1002/path.4633
Dual loss of the SWI/SNF complex ATPases SMARCA4/BRG1 and SMARCA2/BRM is highly sensitive and specific for small cell carcinoma of the ovary, hypercalcaemic type
Abstract
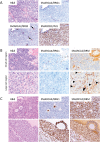
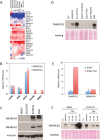
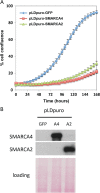
Small cell carcinoma of the ovary, hypercalcaemic type (SCCOHT) is a lethal and sometimes familial ovarian tumour of young women and children. We and others recently discovered that over 90% of SCCOHTs harbour inactivating mutations in the chromatin remodelling gene SMARCA4 with concomitant loss of its encoded protein SMARCA4 (BRG1), one of two mutually exclusive ATPases of the SWI/SNF chromatin remodelling complex. To determine the specificity of SMARCA4 loss for SCCOHT, we examined the expression of SMARCA4 by immunohistochemistry in more than 3000 primary gynaecological tumours. Among ovarian tumours, it was only absent in clear cell carcinoma (15 of 360, 4%). In the uterus, it was absent in endometrial stromal sarcomas (4 of 52, 8%) and high-grade endometrioid carcinomas (2 of 338, 1%). Recent studies have shown that SMARCA2 (BRM), the other mutually exclusive ATPase of the SWI/SNF complex, is necessary for survival of tumour cells lacking SMARCA4. Therefore, we examined SMARCA2 expression and discovered that all SMARCA4-negative SCCOHTs also lacked SMARCA2 protein by IHC, including the SCCOHT cell lines BIN67 and SCCOHT1. Among ovarian tumours, the SMARCA4/SMARCA2 dual loss phenotype appears completely specific for SCCOHT. SMARCA2 loss was not due to mutation but rather from an absence of mRNA expression, which was restored by treatment with the histone deacetylase inhibitor trichostatin A. Re-expression of SMARCA4 or SMARCA2 inhibited the growth of BIN67 and SCCOHT1 cell lines. Our results indicate that SMARCA4 loss, either alone or with SMARCA2, is highly sensitive and specific for SCCOHT and that restoration of either SWI/SNF ATPase can inhibit the growth of SCCOHT cell lines.
Keywords: HDAC inhibitor; SMARCA2/BRM; SMARCA4/BRG1; SMARCB1/INI1; SWI/SNF; epigenetic silencing; hypercalcaemic type; rhabdoid tumour; small cell carcinoma; trichostatin A.
© 2015 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Figures


References
-
- Young RH, Oliva E, Scully RE. Small cell carcinoma of the ovary, hypercalcemic type: a clinicopathological analysis of 150 cases. Am J Surg Pathol 1994; 18 : 1102–1116. - PubMed
-
- Witkowski L, Carrot‐Zhang J, Albrecht S, et al. Germline and somatic SMARCA4 mutations characterize small cell carcinoma of the ovary, hypercalcemic type. Nature Genet 2014; 46 : 438–443. - PubMed
-
- Kupryjanczyk J, Dansonka‐Mieszkowska A, Moes‐Sosnowska J, et al. Ovarian small cell carcinoma of hypercalcemic type – evidence of germline origin and smarca4 gene inactivation. A pilot study. Pol J Pathol 2013; 64: 238–246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

