Chronic Compression of the Dorsal Root Ganglion Enhances Mechanically Evoked Pain Behavior and the Activity of Cutaneous Nociceptors in Mice
- PMID: 26356638
- PMCID: PMC4565551
- DOI: 10.1371/journal.pone.0137512
Chronic Compression of the Dorsal Root Ganglion Enhances Mechanically Evoked Pain Behavior and the Activity of Cutaneous Nociceptors in Mice
Abstract
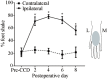
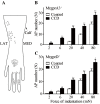
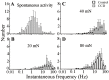
Radicular pain in humans is usually caused by intraforaminal stenosis and other diseases affecting the spinal nerve, root, or dorsal root ganglion (DRG). Previous studies discovered that a chronic compression of the DRG (CCD) induced mechanical allodynia in rats and mice, with enhanced excitability of DRG neurons. We investigated whether CCD altered the pain-like behavior and also the responses of cutaneous nociceptors with unmyelinated axons (C-fibers) to a normally aversive punctate mechanical stimulus delivered to the hairy skin of the hind limb of the mouse. The incidence of a foot shaking evoked by indentation of the dorsum of foot with an aversive von Frey filament (tip diameter 200 μm, bending force 20 mN) was significantly higher in the foot ipsilateral to the CCD surgery as compared to the contralateral side on post-operative days 2 to 8. Mechanically-evoked action potentials were electrophysiologically recorded from the L3 DRG, in vivo, from cell bodies visually identified as expressing a transgenically labeled fluorescent marker (neurons expressing either the receptor MrgprA3 or MrgprD). After CCD, 26.7% of MrgprA3+ and 32.1% MrgprD+ neurons exhibited spontaneous activity (SA), while none of the unoperated control neurons had SA. MrgprA3+ and MrgprD+ neurons in the compressed DRG exhibited, in comparison with neurons from unoperated control mice, an increased response to the punctate mechanical stimuli for each force applied (6, 20, 40, and 80 mN). We conclude that CCD produced both a behavioral hyperalgesia and an enhanced response of cutaneous C-nociceptors to aversive punctate mechanical stimuli.
Conflict of interest statement
Figures
References
-
- Marziniak M, Phan NQ, Raap U, Siepmann D, Schurmeyer-Horst F, Pogatzki-Zahn E, et al. Brachioradial pruritus as a result of cervical spine pathology: the results of a magnetic resonance ctomography study. Journal of the American Academy of Dermatology. 2011;65(4):756–62. 10.1016/j.jaad.2010.07.036 . - DOI - PubMed
-
- Olmarker K, Rydevik B, Nordborg C. Autologous nucleus pulposus induces neurophysiologic and histologic changes in porcine cauda equina nerve roots. Spine. 1993;18(11):1425–32. . - PubMed
-
- Kawakami M, Tamaki T, Weinstein JN, Hashizume H, Nishi H, Meller ST. Pathomechanism of pain-related behavior produced by allografts of intervertebral disc in the rat. Spine. 1996;21(18):2101–7. . - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

