The Discovery and Development of a Potent Antiviral Drug, Entecavir, for the Treatment of Chronic Hepatitis B
- PMID: 26357607
- PMCID: PMC4521267
- DOI: 10.14218/JCTH.2013.00006
The Discovery and Development of a Potent Antiviral Drug, Entecavir, for the Treatment of Chronic Hepatitis B
Abstract
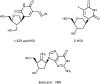
Since the first approval of interferon for the treatment of chronic hepatitis B virus (HBV) infection in 1992, six additional antivirals have been developed: pegylated interferon-alfa2a, and the oral antivirals lamivudine, adefovir, telbivudine, entecavir and tenofovir. The availability of animal models for HBV infection and hepatocyte cell culture led to the discovery and development of oral antivirals targeted at HBV polymerase and reverse transcriptase, which inhibit viral replication. The discovery and development of entecavir, the first oral anti-HBV drug with both potent antiviral activity and a high genetic barrier to resistance, took more than 10 years before it was first approved in the USA. Since then, multiple real-life studies have provided data consistent with the findings of the registration trials and the long-term rollover study in terms of efficacy, resistance, and safety. Data from the long-term follow-up of patients enrolled in the registration studies showed that treatment with entecavir can lead to significant improvements in liver histopathology, and recent cohort studies have demonstrated that treatment with entecavir may reduce disease progression and the development of hepatocellular carcinoma (HCC) in patients with chronic hepatitis B. In addition, real-life studies suggest that entecavir may reduce HCC recurrence and increase survival rates in patients with HBV-related HCC post-surgical resection.
Keywords: Chronic hepatitis B; Development; Discovery; Entecavir; HBV DNA; Hepatitis B Virus; Resistance.
Conflict of interest statement
Figures




References
-
- World Health Organization: Hepatitis B Fact Sheet No. 204. http://www.who.int/mediacentre/factsheets/fs204/en/index.html. & http://www.who.int/immunization/topics/hepatitis_b/en/Accessed November 2012.
-
- Müller R, Baumgarten R, Markus R, Schulz M, Wittenberg H, Hintsche-Kilger B, et al. Treatment of chronic hepatitis B with interferon alfa-2b. J Hepatol. 1990;11(Suppl 1):S137–S140. - PubMed
-
- Deinstag JL. Hepatitis B Virus Infection. N Engl J Med. 2008;359:1486–1500. - PubMed
-
- Lok ASF, McMahon BJ. Chronic Hepatitis B: Update 2009. AASLD Practice Guidelines. Hepatology. 2009;50:1–36.
-
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of chronic hepatitis B. J Hepatol. 2012;57:167–185. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
