Oxidative Reactivities of 2-Furylquinolines: Ubiquitous Scaffolds in Common High-Throughput Screening Libraries
- PMID: 26358009
- PMCID: PMC4610730
- DOI: 10.1021/acs.jmedchem.5b00930
Oxidative Reactivities of 2-Furylquinolines: Ubiquitous Scaffolds in Common High-Throughput Screening Libraries
Abstract
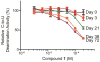
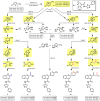
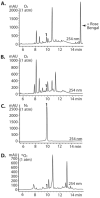
High-throughput screening (HTS) was employed to discover APOBEC3G inhibitors, and multiple 2-furylquinolines (e.g., 1) were found. Dose-response assays with 1 from the HTS sample, as well as commercial material, yielded similar confirmatory results. Interestingly, freshly synthesized and DMSO-solubilized 1 was inactive. Repeated screening of the DMSO aliquot of synthesized 1 revealed increasing APOBEC3G inhibitory activity with age, suggesting that 1 decomposes into an active inhibitor. Laboratory aging of 1 followed by analysis revealed that 1 undergoes oxidative decomposition in air, resulting from a [4 + 2] cycloaddition between the furan of 1 and (1)O2. The resulting endoperoxide then undergoes additional transformations, highlighted by Baeyer-Villager rearrangements, to deliver lactam, carboxylic acid, and aldehyde products. The endoperoxide also undergoes hydrolytic opening followed by further transformations to a bis-enone. Eight structurally related analogues from HTS libraries were similarly reactive. This study constitutes a cautionary tale to validate 2-furylquinolines for structure and stability prior to chemical optimization campaigns.
Figures


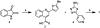
Similar articles
-
First-in-class small molecule inhibitors of the single-strand DNA cytosine deaminase APOBEC3G.ACS Chem Biol. 2012 Mar 16;7(3):506-17. doi: 10.1021/cb200440y. Epub 2012 Jan 17. ACS Chem Biol. 2012. PMID: 22181350 Free PMC article.
-
Discovery of thiadiazole amides as potent, S1P₃-sparing agonists of sphingosine-1-phosphate 1 (S1P₁) receptor.Bioorg Med Chem Lett. 2012 Apr 1;22(7):2456-9. doi: 10.1016/j.bmcl.2012.02.016. Epub 2012 Feb 15. Bioorg Med Chem Lett. 2012. PMID: 22386243
-
T cells contain an RNase-insensitive inhibitor of APOBEC3G deaminase activity.PLoS Pathog. 2007 Sep 21;3(9):1320-34. doi: 10.1371/journal.ppat.0030135. PLoS Pathog. 2007. PMID: 17892323 Free PMC article.
-
Fused Tetrahydroquinolines Are Interfering with Your Assay.J Med Chem. 2023 Nov 9;66(21):14434-14446. doi: 10.1021/acs.jmedchem.3c01277. Epub 2023 Oct 24. J Med Chem. 2023. PMID: 37874947 Free PMC article. Review.
-
Antiviral roles of APOBEC proteins against HIV-1 and suppression by Vif.Arch Virol. 2009;154(10):1579-88. doi: 10.1007/s00705-009-0481-y. Epub 2009 Aug 12. Arch Virol. 2009. PMID: 19669862 Review.
Cited by
-
Screen Targeting Lung and Prostate Cancer Oncogene Identifies Novel Inhibitors of RGS17 and Problematic Chemical Substructures.SLAS Discov. 2018 Apr;23(4):363-374. doi: 10.1177/2472555217752301. Epub 2018 Jan 19. SLAS Discov. 2018. PMID: 29351497 Free PMC article.
-
Modeling Small-Molecule Reactivity Identifies Promiscuous Bioactive Compounds.J Chem Inf Model. 2018 Aug 27;58(8):1483-1500. doi: 10.1021/acs.jcim.8b00104. Epub 2018 Jul 23. J Chem Inf Model. 2018. PMID: 29990427 Free PMC article.
-
Nuisance compounds in cellular assays.Cell Chem Biol. 2021 Mar 18;28(3):356-370. doi: 10.1016/j.chembiol.2021.01.021. Epub 2021 Feb 15. Cell Chem Biol. 2021. PMID: 33592188 Free PMC article. Review.
-
Addressing the benefits of inhibiting APOBEC3-dependent mutagenesis in cancer.Nat Genet. 2022 Nov;54(11):1599-1608. doi: 10.1038/s41588-022-01196-8. Epub 2022 Oct 24. Nat Genet. 2022. PMID: 36280735 Free PMC article. Review.
-
Fragment-Based Nuclear Magnetic Resonance Screen against a Regulator of G Protein Signaling Identifies a Binding "Hot Spot".Chembiochem. 2021 May 4;22(9):1609-1620. doi: 10.1002/cbic.202000740. Epub 2021 Feb 16. Chembiochem. 2021. PMID: 33480159 Free PMC article.
References
-
- Inglese J, Shamu CE, Guy RK. Reporting data from high-throughput screening of small-molecule libraries. Nat Chem Biol. 2007;3:438–441. - PubMed
-
- Baell JB. Observations on screening-based research and some concerning trends in the literature. Future Med Chem. 2010;2:1529–1546. - PubMed
-
- Baell JB, Holloway GA. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J Med Chem. 2010;53:2719–2740. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

