Dressings and securement devices for central venous catheters (CVC)
- PMID: 26358142
- PMCID: PMC6457749
- DOI: 10.1002/14651858.CD010367.pub2
Dressings and securement devices for central venous catheters (CVC)
Abstract
Background: Central venous catheters (CVCs) play a vital role in the management of acute and chronic illness. Dressings and securement devices must ensure CVCs do not dislodge or fall out, provide a barrier protection from microbial colonisation and infection, and be comfortable for the patient. There is a large range of dressing and securement products available for clinicians to use.
Objectives: To compare the available dressing and securement devices for CVCs, in terms of catheter-related bloodstream infection (BSI), catheter colonisation, entry- and exit-site infection, skin colonisation, skin irritation, failed catheter securement, dressing condition and mortality.
Search methods: In June 2015 we searched: The Cochrane Wounds Group Specialised Register; The Cochrane Central Register of Controlled Trials (CENTRAL); The Database of Abstracts of Reviews of Effects (DARE); NHS Economic Evaluation Database (NHSEED); Ovid MEDLINE; Ovid MEDLINE (In-Process & Other Non-Indexed Citations); Ovid EMBASE; EBSCO CINAHL; six clinical trial registries and reference lists of identified trials. There were no restrictions based on language or date of publication or study setting.
Selection criteria: We included randomised controlled trials that evaluated the effects of dressing and securement devices for CVCs. All types of CVCs were included, i.e. short- and long-term CVCs, tunnelled and non-tunnelled, port-a-caths, haemodialysis catheters, and peripherally-inserted central catheters (PICCs).
Data collection and analysis: We used standard Cochrane Collaboration methods including independent review of titles and abstracts for relevance, data extraction, and risk of bias assessment of the included studies by two review authors. Results are expressed using risk ratio (RR) for categorical data with 95% confidence intervals (CIs). For outcomes best presented as a rate-per-time-period, rate ratios and standard errors have been used. We performed multiple treatment meta-analyses to rank the effectiveness of each intervention for each outcome.
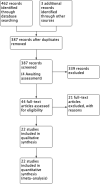
Main results: We included 22 studies involving 7436 participants comparing nine different types of securement device or dressing. All included studies were at unclear or high risk of performance bias due to the different appearances of the dressings and securement devices. The extent of blinding of outcome assessment was unclear in most studies. The quality of evidence varied between different comparisons and outcomes. We mainly downgraded the quality of evidence for imprecision, indirectness, risk of bias and inconsistency.It is unclear whether there is a difference in the rate of catheter-related BSI between securement with gauze and tape and standard polyurethane (SPU) (RR 0.64, 95% CI 0.26 to 1.63, low quality evidence), or between chlorhexidine gluconate-impregnated (CGI) dressings and SPU (RR 0.65, 95% CI 0.40 to 1.05, moderate quality evidence). There is high quality evidence that medication-impregnated dressings reduce the incidence of catheter-related BSI relative to all other dressing types (RR 0.60, 95% CI 0.39 to 0.93).There is moderate quality evidence that CGI dressings reduce the frequency of catheter-related BSI per 1000 patient days compared with SPU dressings (RR 0.51, 95% CI 0.33 to 0.78).There is moderate quality evidence that catheter tip colonisation is reduced with CGI dressings compared with SPU dressings (RR 0.58, 95% CI 0.47 to 0.73), but the relative effects of gauze and tape and SPU are unclear (RR 0.95, 95% CI 0.51 to 1.77, very low quality evidence). It is unclear if there is a difference in rates of skin irritation or damage when CGI dressings are compared with SPU dressings (moderate quality evidence) (RR 11.17, 95% CI 0.84 to 149.48).A multiple treatment meta-analysis found sutureless securement devices as likely to be the most effective at reducing the incidence of catheter-related BSI (low quality evidence), with CGI dressings ranked second (low quality evidence).
Authors' conclusions: Medication-impregnated dressing products reduce the incidence of catheter-related BSI relative to all other dressing types. There is some evidence that CGI dressings, relative to SPU dressings, reduce catheter-related BSI for the outcomes of frequency of infection per 1000 patient days, risk of catheter tip colonisation and possibly risk of catheter-related BSI. A multiple treatment meta-analysis found that sutureless securement devices are likely to be the most effective at reducing catheter-related BSI though this is low quality evidence. Most studies were conducted in intensive care unit (ICU) settings. More, high quality research is needed regarding the relative effects of dressing and securement products for CVCs. Future research may adjust the estimates of effect for the products included in this review and is needed to assess the effectiveness of new products.
Conflict of interest statement
Griffith University received an unrestricted educational grant from 3M (a manufacturer of CVC dressings and securement devices) to assist with the costs of Claire Rickard's (CR) research nurses' travel to a conference in 2014. Griffith University received a consultancy payment from 3M in 2013 for CR to present an educational lecture based on her independent research. Griffith University received an unrestricted investigator initiated research grant from 3M in 2014 to support a research study on which CR is an investigator, but this study was unrelated to CVC dressings and securement. Studies involving 3M's CVC dressings (Tegaderm range) are included in this review but the conclusions do not recommend these over competitor products. Griffith University received an unrestricted investigator‐initiated research grant from Centurion Medical (manufacturer of CVC dressings) as part‐funding for the CASCADE Pilot Trial led by CR, and for a PhD student scholarship Top Up for Amanda J Ullman. No trials investigating Centurion Medical's dressings were included in this review.
Marie L Cooke: nothing to declare Marion Mitchell: nothing to declare Frances Lin: nothing to declare Karen New: has received a research grant from The University of Queensland for research related to preconception and early pregnancy care; has been paid for preparing and delivering education on obtaining informed consent for research; and as the Professional Officer of the Australian College of Neonatal Nurses has been supported by an unrestricted eduction grant from Johnson and Johnson Pacific to deliver education on the AWHONN skin care guidelines and the Every Newborn Action Plan. Debbie A Long: nothing to declare Gabor Mihala: nothing to declare
Figures










Update of
References
References to studies included in this review
Arvaniti 2012 {published data only}
-
- Arvaniti K, Lathyris D, Clouva‐Molyvdas P, Haidich AB, Mouloudi E, Synnefaki E, et al. Comparison of oligon catheters and chlorhexidine impregnated sponges with standard multilumen central venous catheters for prevention of associated colonization and infections in intensive care unit patients: a multicenter, randomized, controlled trial. Critical Care Medicine 2012;40(2):420‐9. [PUBMED: 21926583] - PubMed
Brandt 1996 {published data only}
-
- Brandt B, DePalma J, Irwin M, Shogan J, Lucke J. Comparison of central venous cathter dressings in bone marrow transplant recipients. Oncology Nursing Forum 1996;23(5):829‐36. [PUBMED: 8792352] - PubMed
Carrer 2005 {published data only}
-
- Carrer S, Bocchi A, Bortolotti M, Braga N, Gilli G, Candini M, et al. Effect of different sterile barrier precautions and central venous catheter dressing on the skin colonization around the insertion site. Minerva Anestesiologica 2005;71(5):197‐206. [PUBMED: 15834348] - PubMed
Chambers 2005 {published data only}
-
- Chambers S, Sanders J, Patton W, Ganly P, Birch M, Crump J, et al. Reduction of exit‐site infections of tunnelled intravascular catheters among neutropenic patients by sustained‐release chlorhexidine dressings: results from a prospective randomized controlled trial. Journal of Hospital Infection 2005;61:53‐61. [PUBMED: 16002181] - PubMed
Conly 1989 {published data only}
-
- Conly JM, Grieves K, Peters B. A prospective, randomized study comparing transparent and dry gauze dressings for central venous catheters. The Journal of Infectious Diseases 1989;159(2):310‐9. [PUBMED: 2644372] - PubMed
de Barros 2009 {published data only}
-
- Barros L, Arenas V, Bettencourt A, Diccini S, Fram D, Belasco A, et al. Evaluation of two types of dressings used on central venous catheters for hemodialysis. Acta Paulista de Enfermagem 2009;22(Especial ‐ Nefrologia):481‐6.
Garland 2001 {published data only}
-
- Garland J, Alex C, Mueller C, Otten D, Shivpuri C, Harris M, et al. A randomized trial comparing povidone‐iodine to a chlorhexidine gluconate‐impregnated dressing for prevention of central venous catheter infections in neonates. Pediatrics 2001;107(6):1431‐7. [PUBMED: 11389271] - PubMed
Giles 2002 {published data only}
-
- Giles Y, Aksoy A, Tezelman S. What really affects the incidence of central venous catheter‐related infections for short‐term catheterization. Acta Chirugica Belgica 2002;102(4):256‐8. [PUBMED: 12244905] - PubMed
Hagerstrom 1994 {published data only}
-
- Hagerstrom M, Matthiesen K, Thuessen C. [An improved dressing method for haemodialysis patients with permanent central venous catheters]. Associazione Infermieristica per lo Studio delle Lesioni Cutanee. 1994. [http://www.aislec.it/download2010/1190.pdf]
Hill 2010 {published data only}
-
- Hill M, Baldwin L, Slaughter J, Walsh W, Weitkamp J. A silver‐alginate‐coated dressing to reduce peripherally inserted central catheter infections in NICU patients: a pilot randomized controlled trial. Journal of Perinatology 2010;30:469‐73. [PUBMED: 20010613] - PubMed
le Corre 2003 {published data only}
-
- Corre L, Delorme M, Cournoyer S. A prospective, randomized trial comparing a transparent dressing and a dry gauze on the exit site of long term central venous catheters of hemodialysis patients. The Journal of Vascular Access 2003;4(2):56‐61. [PUBMED: 17642061] - PubMed
Levy 2005 {published and unpublished data}
-
- Levy I, Katz J, Solter E, Samra Z, Vidne B, Birk E, et al. Chlorhexidine‐impregnated dressing for prevention of colonization of central venous catheters in infants and children. Pediatric Infectious Disease Journal 2005;24(8):676‐9. [PUBMED: 16094219] - PubMed
Nikoletti 1999 {published and unpublished data}
-
- Nikoletti S, Leslie G, Gandossi S, Coombs G, Wilson R. A prospective, randomized, controlled trial comparing transparent polyurethane and hydrocolloid dressings for central venous catheters. American Journal of Infection Control 1999;27(6):488‐96. [PUBMED: 10586152] - PubMed
Olson 2004 {published and unpublished data}
-
- Olson K, Rennie R, Hanson J, Ryan M, Gilpin J, Falsetti M, et al. Evaluation of a no‐dressing intervention for tunneled central venous catheter exit sites. Journal of Infusion Nursing 2004;27(1):37‐55. [PUBMED: 14734986] - PubMed
Pedrolo 2011 {published and unpublished data}
-
- Pedrolo E, Danski M, Mingorance P, Lazzari L, Johann D. Clinical controlled trial on central venous catheter dressings. Acta Paulista de Enfermagem 2011;24(2):278‐83.
Roberts 1998 {published data only}
-
- Roberts B, Cheung D. Biopatch ‐ a new concept in antimicrobial dressings for invasive devices. Australia Critical Care 1998;11(1):16‐9. [PUBMED: 9708081] - PubMed
Ruschulte 2009 {published data only}
-
- Ruschulte H, Franke M, Gastmeier P, Zenz S, Nahr K, Buchholz S, et al. Prevention of central venous catheter related infections with chlorhexidine gluconate impregnated wound dressings: a randomized controlled trial. Annals of Hematology 2009;88:267‐72. [PUBMED: 18679683] - PubMed
Shivnan 1991 {published data only}
-
- Shivnan J, McGuire D, Freedman S, Sharkazy E, Bosserman G, Larson E, et al. A comparison of transparent adherent and dry sterile gauze dressings for long‐term central catheters in patients undergoing bone marrow transplant. Oncology Nursing Forum 1991;18(8):1349‐56. [PUBMED: 1762975] - PubMed
Timsit 2009 {published and unpublished data}
-
- Roush K. Drug‐impregnated sponges for preventing catheter‐related infections. American Journal of Nursing 2009;109(11):65. [PUBMED: 19858861] - PubMed
-
- Timsit JF, Schwebel C, Duoadma L, Geffroy M, Pease S, Herault MC, et al. Chlorhexidine‐impregnated sponges and less frequent dressing changes for prevention of catheter‐related infections in critically ill adults. JAMA 2009;301(12):1231‐40. [PUBMED: 19318651] - PubMed
Timsit 2012 {published and unpublished data}
-
- Timsit JF, Mimoz O, Mourvillier B, Souweine B, Garrouste‐Orgeas M, Alfandari S, et al. Randomized controlled trial of chlorhexidine dressing and highly adhesive dressing for preventing catheter‐related infections in critically ill adults. American Journal of Respiratory and Critical Care Medicine 2012;186(12):1272‐8. [PUBMED: 23043083] - PubMed
Wille 1993 {published data only}
-
- Wille J, Blusse van Oud Alblas A, Thewessen E. A comparison of two transparent film‐type dressings in central venous therapy. Journal of Hospital Infection 1993;23:113‐21. [PUBMED: 8097215] - PubMed
Yamamoto 2002 {published and unpublished data}
-
- Yamamoto A, Solomon J, Soulen M, Tang J, Parkinson K, Lin R, et al. Sutureless securement device reduces complications of peripherally inserted central venous catheters. Journal of Vascular and Interventional Radiology 2002;13:77‐81. [PUBMED: 11788698] - PubMed
References to studies excluded from this review
Crawford 2004 {published data only}
-
- Crawford A, Fuhr J, Rao B. Cost‐benefit analysis of chlorhexidine gluconate dressing in the prevention of catheter‐related bloodstream infection. Infection Control and Hospital Epidemiology 2004;25(8):668‐74. [PUBMED: 15357159] - PubMed
Davidson 1986 {published data only}
-
- Davidson L. Dressing subclavian catheters. Nursing Times 1986;82(7):40. [PUBMED: 3083404] - PubMed
Freiberger 1992 {published data only}
-
- Freiberger D, Bryant J, Marino B. The effects of different central venous line dressing changes on bacterial growth in a pediatric oncology population. Journal of Pediatric Oncology Nursing 1992;9(1):3‐6. [PUBMED: 1596385] - PubMed
George 2011 {published data only}
-
- George J, Singh S, Deepshikha, John M. A prospective randomized comparative study between transparent and occlusive dressings for central venous catheters. Indian Journal of Hematology and Blood Transfusion. 2011; Vol. 27 (4):286.
Keenlyside 1991 {published data only}
-
- Keenlyside D. Central venous catheters ‐ a randomized comparative study of OpSite IV3000 and Tegaderm. In: Maki D editor(s). Improving Catheter Site Care. Royal Society of Medicine Services Limited, 1991:47‐50.
Keenlyside 1993 {published data only}
-
- Kennlyside D. Avoiding an unnecessary outcome: a comparative trial between IV3000 and a conventional film dressing to assess rates of catheter‐related sepsis. Professional Nurse 1993;8(5):288‐91. [PUBMED: 8451247] - PubMed
Khattak 2010 {published data only}
-
- Khattak A, Ross R, Ngo T, Shoemaker C. A randomized controlled evaluation of absorption of silver with the use of silver alginate (Algidex) patches in very low birth weight (VBLW) infants with central lines. Journal of Perinatology 2010;30:337‐42. [PUBMED: 19940856] - PubMed
Lawson 1986 {published data only}
-
- Lawson M, Kavanagh T, McCredie K, Marts K, Barbour N, Chandler W. Comparison to transparent dressing to paper tape dressing over central venous catheter sites. Journal of the National Intravenous Therapy Association 1986;9:40‐3. [PUBMED: 3633416 ] - PubMed
Little 1998 {published data only}
-
- Little K, Palmer D. Central line exit sites: which dressing?. Nursing Standard 1998;12(48):42‐6. [PUBMED: 9823178] - PubMed
Lucas 1996 {published data only}
-
- Lucas H, Attard‐Montalto S. Central line dressings: study of infection rates. Paediatric Nursing 1996;8(6):21‐3. [PUBMED: 8718254] - PubMed
Madeo 1998 {published data only}
-
- Madeo M, Martin C, Turner C, Kirkby V, Thompson D. A randomized trial comparing Arglaes (a transparent dressing containing silver ions) to Tegaderm (a transparent polyurethane dressing) for dressing peripheral arterial catheters and central vascular catheters. Intensive and Critical Care Nursing 1998;14:187‐91. [PUBMED: 9849245] - PubMed
Maki 1984 {published and unpublished data}
-
- Maki D, Wili L. Colonisation and infection associated with transparent dressings for central venous arterial and hickman catheters: a comparative trial. Program and Abstracts of the 24th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, DC, 1984.
Maki 2000 {published data only (unpublished sought but not used)}
-
- Maki DG, Mermel LA, Kluger D, Narans L, Knasinski V, Parenteau S, et al. The efficacy of a chlorhexidine‐impregnated sponge (biopatch) for the prevention of intravascular catheter‐related infection ‐ a prospective, randomized, controlled, multicenter study. Abstracts of the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy. 2000:422.
Neufeld 1991 {published and unpublished data}
-
- Neufeld M. A randomized control trial of the effectiveness of opsite wound versus IV 3000 in maintaining an occlusive central line dressing. Open Access Dissertations and Theses 1991; Vol. Paper 4057. [http://Digitalcommons.mcmaster.ca/opendissertations/4057]
Olson 2008 {published data only}
-
- Olson C, Heilman J and the Vascular Access Team of Abbott Northwestern Hospital. Clinical performance of a new transparent chlorhexidine gluconate central venous catheter dressing. Journal for the Association for Vascular Access 2008;13(1):13‐6.
Petrosino 1988 {published data only}
-
- Petrosino B, Becker H, Christian B. Infection rates in central venous catheter dressings. Oncology Nursing Forum 1988;15(6):709‐17. [PUBMED: 3144709] - PubMed
Powell 1982 {published data only}
-
- Powell C, Regan C, Fabri P, Ruberg R. Evaluation of opsite catheter dressings for parenteral nutrition: a prospective, randomized study. Journal of Parenteral and Enteral Nutrition 1982;6(1):43‐6. [PUBMED: 6804654] - PubMed
Powell 1985 {published data only}
-
- Powell C, Traetow M, Fabri P, Kudsk K, Ruberg R. Op‐site dressing study: a prospective randomized study evaluating povidine iodine ointment and extension set changes with 7‐day Op‐site dressings applied to total parental nutrition subclavian sites. Journal of Parenteral and Enteral Nutrition 1985;9(4):443‐8. [PUBMED: 3928918] - PubMed
Reynolds 1997 {published data only}
-
- Reynolds M, Tebbs S, Elliot T. Do dressings with increased permeability reduce the incidence of central venous catheter related sepsis?. Intensive and Critical Care Nursing 1997;13:26‐9. [PUBMED: 9095879] - PubMed
Schwebel 2012 {published data only}
-
- Schwebel D, Lucet JC, Vesin A, Arrault X, Calvino‐Gunther S, Bouadma L, et al. Economic evaluation of chlorhexidine‐impregnated sponges for preventing catheter‐related infections in critically ill adults in the Dressing Study. Critical Care Medicine 2012;40(1):11‐8. [PUBMED: 21926570] - PubMed
Timsit 2010 {published data only}
-
- Timsit JF, Schwebel C, Vegin A, Bouadma L, Geffroy A, Garrouste‐Orgeas M, et al. Cost‐benefit of a chlorhexidine impregnated sponges for prevention of catheter‐related infections in adult ICU patients. 23rd ESICM Annual Congress. 2010:S207.
References to studies awaiting assessment
Broadhurst 2014 {published data only}
-
- Broadhurst D. PICC catheter securement: a randomized controlled trial in the home care setting. Journal of Vascular Access. 2015; Vol. 15:218‐9.
Calvino 2014 {published data only}
-
- Calvino GS, Chautemps M, Schwebel C, Sengel E, Ruckly S, Djaguidi MR, et al. Performance of new high performance dressings as compared to traditional ones in prevention infection and non‐infection complications of catheters in ICU: a randomized controlled study. Intensive Care Medicine. 2014:S240.
Gu 2014 {published data only}
-
- Gu Y, Yuan J, Hu J, Gong WJ. Transparent dressing versus iodine plus dressing and high‐permeability dressing in pediatric central line maintenance: a randomize controlled study. Pediatric Critical Care Medicine. 2014.
Additional references
Chaimani 2013
Deeks 2011
-
- Deeks J, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Egger 1997
Frasca 2010
Han 2010
Higgins 2003
Higgins 2011a
-
- Higgins JPT, Altman DG, Stern JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
-
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ho 2006
-
- Ho KM, Litton E. Use of chlorhexidine‐impregnated dressing to prevent vascular and epidural catheter colonization and infection: a meta‐analysis. The Journal of Antimicrobial Chemotherapy 2006;58(2):281‐7. [PUBMED: 16757502] - PubMed
Kampf 2005
-
- Kampf G, Wigger‐Alberti W, Schoder V, Wilhelm K. Emollients in a propanol‐based hand rub can significantly decrease irritant contact dermatitis. Contact Dermatitis: Environmental and Occupational Dermatitis 2005;53(6):344‐9. [PUBMED: 16364124 ] - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville F. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The CochraneCollaboration, 2011. Available from www.cochrane‐handbook.org.
Liberati 2009
Loveday 2014
Maki 2006
-
- Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clinic Proceedings 2006;81(9):1159‐71. - PubMed
Mermel 2009
Naimer 2004
-
- Naimer S, Temira F. Evaluation of techniques for intravenous catheter and tubing fixation. Military Medicine 2004;169(1):79‐81. - PubMed
O'Grady 2002
-
- O'Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, et al. Guidelines for the prevention of intravascular catheter‐related infections. Morbidity and Mortality Weekly Report 2002;51(RR‐10):1‐29. - PubMed
O'Grady 2011
Parmar 1998
-
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. [PUBMED: 9921604] - PubMed
Pronovost 2006
-
- Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, et al. An intervention to decrease catheter‐related bloodstream infections in the ICU. New England Journal of Medicine 2006;355(26):2725‐32. - PubMed
Raad 2007
-
- Raad I, Hanna H, Maki D. Intravascular catheter‐related infections: advances in diagnosis, prevention, and management. Lancet 2007;7:645‐57. - PubMed
Reeves 2011
-
- Reeves BC, Deeks JJ, Higgins JPT, Wells GA. Chapter 13: Including non‐randomized studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Safdar 2014
Salanti 2008
-
- Salanti G, Higgins J, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Statistical Methods in Medical Research 2008;17(3):279‐301. - PubMed
Salanti 2014
Schulz 2010
-
- Schulz KF, Altman DF, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. International Journal of Surgery 2011;9(8):672‐7. [PUBMED: 22019563] - PubMed
Schunemann 2011
-
- Schunemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Hanbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www. cochrane‐handbook.org.
SIGN 2012
-
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. www.sign.ac.uk/methodology/filters.html#random (accessed 19 November 2012).
StataCorp 2011 [Computer program]
-
- StataCorp. Stata Statistical Software: Release 12. College Station TX: StataCorp LP, 2011.
Timsit 2011
Webster 2011
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

