Acute inflammation stimulates a regenerative response in the neonatal mouse heart
- PMID: 26358185
- PMCID: PMC4650627
- DOI: 10.1038/cr.2015.110
Acute inflammation stimulates a regenerative response in the neonatal mouse heart
Abstract
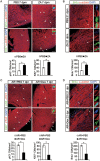
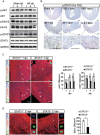
Cardiac injury in neonatal 1-day-old mice stimulates a regenerative response characterized by reactive cardiomyocyte proliferation, which is distinguished from the fibrotic repair process in adults. Acute inflammation occurs immediately after heart injury and has generally been believed to exert a negative effect on heart regeneration by promoting scar formation in adults; however, little is known about the role of acute inflammation in the cardiac regenerative response in neonatal mice. Here, we show that acute inflammation induced cardiomyocyte proliferation after apical intramyocardial microinjection of immunogenic zymosan A particles into the neonatal mouse heart. We also found that cardiac injury-induced regenerative response was suspended after immunosuppression in neonatal mice, and that cardiomyocytes could not be reactivated to proliferate after neonatal heart injury in the absence of interleukin-6 (IL-6). Furthermore, cardiomyocyte-specific deletion of signal transducer and activator of transcription 3 (STAT3), the major downstream effector of IL-6 signaling, decreased reactive cardiomyocyte proliferation after apical resection. Our results indicate that acute inflammation stimulates the regenerative response in neonatal mouse heart, and suggest that modulation of inflammatory signals might have important implications in cardiac regenerative medicine.
Figures




References
-
- 1Ptaszek LM, Mansour M, Ruskin JN, Chien KR. Towards regenerative therapy for cardiac disease. Lancet 2012; 379:933–942. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

