Frontotemporal dementia caused by CHMP2B mutation is characterised by neuronal lysosomal storage pathology
- PMID: 26358247
- PMCID: PMC4575387
- DOI: 10.1007/s00401-015-1475-3
Frontotemporal dementia caused by CHMP2B mutation is characterised by neuronal lysosomal storage pathology
Abstract
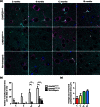
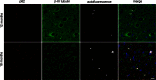
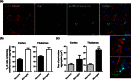
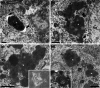
Mutations in the charged multivesicular body protein 2B (CHMP2B) cause frontotemporal dementia (FTD). We report that mice which express FTD-causative mutant CHMP2B at physiological levels develop a novel lysosomal storage pathology characterised by large neuronal autofluorescent aggregates. The aggregates are an early and progressive pathology that occur at 3 months of age and increase in both size and number over time. These autofluorescent aggregates are not observed in mice expressing wild-type CHMP2B, or in non-transgenic controls, indicating that they are a specific pathology caused by mutant CHMP2B. Ultrastructural analysis and immuno- gold labelling confirmed that they are derived from the endolysosomal system. Consistent with these findings, CHMP2B mutation patient brains contain morphologically similar autofluorescent aggregates. These aggregates occur significantly more frequently in human CHMP2B mutation brain than in neurodegenerative disease or age-matched control brains. These data suggest that lysosomal storage pathology is the major neuronal pathology in FTD caused by CHMP2B mutation. Recent evidence suggests that two other genes associated with FTD, GRN and TMEM106B are important for lysosomal function. Our identification of lysosomal storage pathology in FTD caused by CHMP2B mutation now provides evidence that endolysosomal dysfunction is a major degenerative pathway in FTD.
Keywords: CHMP2B; ESCRT; FTD; Lysosomal storage disorder; Lysosome.
Figures


References
-
- Ahmed Z, Sheng H, Xu YF, Lin WL, Innes AE, Gass J, Yu X, Hou H, Chiba S, Yamanouchi K, Leissring M, Petrucelli L, Nishihara M, Hutton ML, McGowan E, et al. Accelerated lipofuscinosis and ubiquitination in granulin knockout mice suggest a role for progranulin in successful aging. Am J Pathol. 2010;177:311–324. doi: 10.2353/ajpath.2010.090915. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

