Cardiac myosin-binding protein C (MYBPC3) in cardiac pathophysiology
- PMID: 26358504
- PMCID: PMC6660134
- DOI: 10.1016/j.gene.2015.09.008
Cardiac myosin-binding protein C (MYBPC3) in cardiac pathophysiology
Abstract
More than 350 individual MYPBC3 mutations have been identified in patients with inherited hypertrophic cardiomyopathy (HCM), thus representing 40–50% of all HCM mutations, making it the most frequently mutated gene in HCM. HCM is considered a disease of the sarcomere and is characterized by left ventricular hypertrophy, myocyte disarray and diastolic dysfunction. MYBPC3 encodes for the thick filament associated protein cardiac myosin-binding protein C (cMyBP-C), a signaling node in cardiac myocytes that contributes to the maintenance of sarcomeric structure and regulation of contraction and relaxation. This review aims to provide a succinct overview of how mutations in MYBPC3 are considered to affect the physiological function of cMyBP-C, thus causing the deleterious consequences observed inHCM patients. Importantly, recent advances to causally treat HCM by repairing MYBPC3 mutations by gene therapy are discussed here, providing a promising alternative to heart transplantation for patients with a fatal form of neonatal cardiomyopathy due to bi-allelic truncating MYBPC3 mutations.
Keywords: Cardiac myosin-binding protein C; Cardiomyopathies; Gene therapy; Hypertrophy postranslational modifications; MYBPC3.
Conflict of interest statement
Conflict of interest statement
The University Medical Center Hamburg-Eppendorf has filed a patent in the area of
Figures


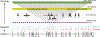
References
-
- Ababou A, Gautel M and Pfuhl M, 2007. Dissecting the N-terminal myosin binding site of human cardiac myosin-binding protein C. Structure and myosin binding of domain C2. J Biol Chem 282, 9204–15. - PubMed
-
- Adalsteinsdottir B, Teekakirikul P, Maron BJ, Burke MA, Gudbjartsson DF, Holm H, Stefansson K, DePalma SR, Mazaika E, McDonough B, Danielsen R, Seidman JG, Seidman CE and Gunnarsson GT, 2014. Nationwide study on hypertrophic cardiomyopathy in Iceland: evidence of a MYBPC3 founder mutation. Circulation 130, 1158–67. - PubMed
-
- Authors/Task Force, m., Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C and Watkins H, 2014. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 35, 2733–79. - PubMed
-
- Bahrudin U, Morisaki H, Morisaki T, Ninomiya H, Higaki K, Nanba E, Igawa O, Takashima S, Mizuta E, Miake J, Yamamoto Y, Shirayoshi Y, Kitakaze M, Carrier L and Hisatome I, 2008. Ubiquitin-proteasome system impairment caused by a missense cardiac myosin-binding protein C mutation and associated with cardiac dysfunction in hypertrophic cardiomyopathy. J Mol Biol 384, 896–907. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

