Plasticity of gene-regulatory networks controlling sex determination: of masters, slaves, usual suspects, newcomers, and usurpators
- PMID: 26358957
- PMCID: PMC4766460
- DOI: 10.15252/embr.201540667
Plasticity of gene-regulatory networks controlling sex determination: of masters, slaves, usual suspects, newcomers, and usurpators
Abstract
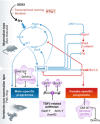
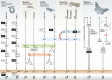
Sexual dimorphism is one of the most pervasive and diverse features of animal morphology, physiology, and behavior. Despite the generality of the phenomenon itself, the mechanisms controlling how sex is determined differ considerably among various organismic groups, have evolved repeatedly and independently, and the underlying molecular pathways can change quickly during evolution. Even within closely related groups of organisms for which the development of gonads on the morphological, histological, and cell biological level is undistinguishable, the molecular control and the regulation of the factors involved in sex determination and gonad differentiation can be substantially different. The biological meaning of the high molecular plasticity of an otherwise common developmental program is unknown. While comparative studies suggest that the downstream effectors of sex-determining pathways tend to be more stable than the triggering mechanisms at the top, it is still unclear how conserved the downstream networks are and how all components work together. After many years of stasis, when the molecular basis of sex determination was amenable only in the few classical model organisms (fly, worm, mouse), recently, sex-determining genes from several animal species have been identified and new studies have elucidated some novel regulatory interactions and biological functions of the downstream network, particularly in vertebrates. These data have considerably changed our classical perception of a simple linear developmental cascade that makes the decision for the embryo to develop as male or female, and how it evolves.
Keywords: Dmrt1; SRY; ovary; testis; transcription factor.
© 2015 The Authors.
Figures

References
-
- Graham P, Penn JK, Schedl P (2003) Masters change, slaves remain. BioEssays 25: 1–4 - PubMed
-
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell‐Badge R (1991) Male development of chromosomally female mice transgenic for Sry. Nature 351: 117–121 - PubMed
-
- Lovell‐Badge R, Robertson E (1990) XY female mice resulting from a heritable mutation in the primary testis‐determining gene, Tdy. Development 109: 635–646 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

