Impact of baseline anti-cyclic citrullinated peptide-2 antibody concentration on efficacy outcomes following treatment with subcutaneous abatacept or adalimumab: 2-year results from the AMPLE trial
- PMID: 26359449
- PMCID: PMC4819608
- DOI: 10.1136/annrheumdis-2015-207942
Impact of baseline anti-cyclic citrullinated peptide-2 antibody concentration on efficacy outcomes following treatment with subcutaneous abatacept or adalimumab: 2-year results from the AMPLE trial
Abstract
Objectives: To examine whether baseline anti-cyclic citrullinated peptide-2 (CCP2) antibody status and concentration correlated with clinical outcomes in patients treated with abatacept or adalimumab on background methotrexate (MTX) in the 2-year AMPLE (Abatacept versus adaliMumab comParison in bioLogic-naïvE rheumatoid arthritis subjects with background MTX) study.
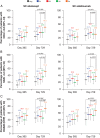
Methods: In this exploratory analysis, anti-CCP2 antibody concentration was measured at baseline, and antibody-positive patients were divided into equal quartiles, Q1-Q4, representing increasing antibody concentrations. Clinical outcomes analysed by baseline anti-CCP2 status and quartile included change from baseline in disease activity and disability and remission rates.
Results: Baseline characteristics were generally comparable across quartiles and treatment groups. In both treatment groups, anti-CCP2 antibody-negative patients responded less well than antibody-positive patients. At year 2, improvements in disease activity and disability and remission rates were similar across Q1-Q3, but were numerically higher in Q4 in the abatacept group; in contrast, treatment effects were similar across all quartiles in the adalimumab group.
Conclusions: In AMPLE, baseline anti-CCP2 positivity was associated with a better response for abatacept and adalimumab. Patients with the highest baseline anti-CCP2 antibody concentrations had better clinical response with abatacept than patients with lower concentrations, an association that was not observed with adalimumab.
Trial registration number: NCT00929864.
Keywords: Ant-CCP; Autoantibodies; DMARDs (biologic); Rheumatoid Arthritis.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

