Generating neuronal diversity in the mammalian cerebral cortex
- PMID: 26359774
- PMCID: PMC4778709
- DOI: 10.1146/annurev-cellbio-100814-125353
Generating neuronal diversity in the mammalian cerebral cortex
Abstract
The neocortex is the part of the brain responsible for execution of higher-order brain functions, including cognition, sensory perception, and sophisticated motor control. During evolution, the neocortex has developed an unparalleled neuronal diversity, which still remains partly unclassified and unmapped at the functional level. Here, we broadly review the structural blueprint of the neocortex and discuss the current classification of its neuronal diversity. We then cover the principles and mechanisms that build neuronal diversity during cortical development and consider the impact of neuronal class-specific identity in shaping cortical connectivity and function.
Keywords: cortical networks; identity; interneurons; myelination profile; neocortex; projection neurons.
Figures
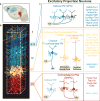
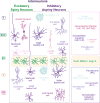

References
-
- Aboitiz F, Morales D, Montiel J. The evolutionary origin of the mammalian isocortex: towards an integrated developmental and functional approach. Behav Brain Sci. 2003;26(5):535–52. discussion 552–85. - PubMed
-
- Alcamo EA, Chirivella L, Dautzenberg M, Dobreva G, Farinas I, et al. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron. 2008;57(3):364–77. - PubMed
-
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278(5337):474–76. - PubMed
-
- Angevine JBJ, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in mouse. Nature. 1961;192:766–68. - PubMed
-
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45(2):207–21. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

