Melanoma Cell-Intrinsic PD-1 Receptor Functions Promote Tumor Growth
- PMID: 26359984
- PMCID: PMC4700833
- DOI: 10.1016/j.cell.2015.08.052
Melanoma Cell-Intrinsic PD-1 Receptor Functions Promote Tumor Growth
Abstract
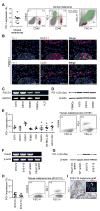
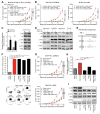
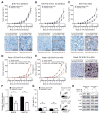
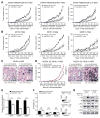
Therapeutic antibodies targeting programmed cell death 1 (PD-1) activate tumor-specific immunity and have shown remarkable efficacy in the treatment of melanoma. Yet, little is known about tumor cell-intrinsic PD-1 pathway effects. Here, we show that murine and human melanomas contain PD-1-expressing cancer subpopulations and demonstrate that melanoma cell-intrinsic PD-1 promotes tumorigenesis, even in mice lacking adaptive immunity. PD-1 inhibition on melanoma cells by RNAi, blocking antibodies, or mutagenesis of melanoma-PD-1 signaling motifs suppresses tumor growth in immunocompetent, immunocompromised, and PD-1-deficient tumor graft recipient mice. Conversely, melanoma-specific PD-1 overexpression enhances tumorigenicity, as does engagement of melanoma-PD-1 by its ligand, PD-L1, whereas melanoma-PD-L1 inhibition or knockout of host-PD-L1 attenuate growth of PD-1-positive melanomas. Mechanistically, the melanoma-PD-1 receptor modulates downstream effectors of mTOR signaling. Our results identify melanoma cell-intrinsic functions of the PD-1:PD-L1 axis in tumor growth and suggest that blocking melanoma-PD-1 might contribute to the striking clinical efficacy of anti-PD-1 therapy.
Keywords: Melanoma; PD-1; PD-L1; S6 ribosomal protein; antibody; blockade; immune checkpoint; mTOR signaling; p-S6; programmed cell death-1; therapy.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures



References
-
- Aceto N, Sausgruber N, Brinkhaus H, Gaidatzis D, Martiny-Baron G, Mazzarol G, Confalonieri S, Quarto M, Hu G, Balwierz PJ, et al. Tyrosine phosphatase SHP2 promotes breast cancer progression and maintains tumor-initiating cells via activation of key transcription factors and a positive feedback signaling loop. Nat Med. 2012;18:529–537. - PubMed
-
- Civenni G, Walter A, Kobert N, Mihic-Probst D, Zipser M, Belloni B, Seifert B, Moch H, Dummer R, van den Broek M, et al. Human CD271-positive melanoma stem cells associated with metastasis establish tumor heterogeneity and long-term growth. Cancer Res. 2011;71:3098–3109. - PubMed
-
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. - PubMed
-
- Flaherty KT, Hodi FS, Fisher DE. From genes to drugs: targeted strategies for melanoma. Nat Rev Cancer. 2012;12:349–361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

