Structural and functional analysis of endosomal compartments in epithelial cells
- PMID: 26360040
- PMCID: PMC5755384
- DOI: 10.1016/bs.mcb.2015.06.019
Structural and functional analysis of endosomal compartments in epithelial cells
Abstract
Epithelial cells display segregated early endosomal compartments, termed apical sorting endosomes and basolateral sorting endosomes, that converge into a common late endosomal-lysosomal degradative compartment and common recycling endosomes (CREs). Unlike recycling endosomes of nonpolarized cells, CREs have the ability to sort apical and basolateral plasma membrane proteins into distinct apical and basolateral recycling routes, utilizing mechanisms similar to those employed by the trans Golgi network in the biosynthetic pathway. The apical recycling route includes an additional compartment, the apical recycling endosomes, consisting of multiple vesicles bundled around the basal body. Recent evidence indicates that, in addition to their role in internalizing ligands and recycling their receptors back to the cell surface, endosomal compartments act as intermediate stations in the biosynthetic routes to the plasma membrane. Here we review methods employed by our laboratory to study the endosomal compartments of epithelial cells and their multiple trafficking roles.
Keywords: Endocytosis; Endosomes; Epithelial cells; Recycling; Transcytosis; Transferrin receptor.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

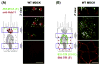
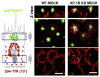
References
-
- Brown DA, Crise B, Rose JK. Mechanism of membrane anchoring affects polarized expression of two proteins in MDCK cells. Science. 1989;245:1499–1501. - PubMed
-
- Brown PS, Wang E, Aroeti B, Chapin SJ, Mostov KE, Dunn KW. Definition of distinct compartments in polarized Madin-Darby canine kidney (MDCK) cells for membrane-volume sorting, polarized sorting and apical recycling. Traffic. 2000;1:124–140. - PubMed
-
- Carvajal-Gonzalez JM, Gravotta D, Mattera R, Diaz F, Perez Bay A, Roman AC, et al. Basolateral sorting of the coxsackie and adenovirus receptor through interaction of a canonical YXXPhi motif with the clathrin adaptors AP-1A and AP-1B. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:3820–3825. - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

