Wittig derivatization of sesquiterpenoid polygodial leads to cytostatic agents with activity against drug resistant cancer cells and capable of pyrrolylation of primary amines
- PMID: 26360047
- PMCID: PMC4617783
- DOI: 10.1016/j.ejmech.2015.08.047
Wittig derivatization of sesquiterpenoid polygodial leads to cytostatic agents with activity against drug resistant cancer cells and capable of pyrrolylation of primary amines
Abstract
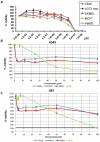
Many types of cancer, including glioma, melanoma, non-small cell lung cancer (NSCLC), among others, are resistant to proapoptotic stimuli and thus poorly responsive to current therapies based on the induction of apoptosis in cancer cells. The current investigation describes the synthesis and anticancer evaluation of unique C12-Wittig derivatives of polygodial, a sesquiterpenoid dialdehyde isolated from Persicaria hydropiper (L.) Delabre. These compounds were found to undergo an unprecedented pyrrole formation with primary amines in a chemical model system, a reaction that could be relevant in the biological environment and lead to the pyrrolation of lysine residues in the target proteins. The anticancer evaluation of these compounds revealed their promising activity against cancer cells displaying various forms of drug resistance, including resistance to proapoptotic agents. Mechanistic studies indicated that compared to the parent polygodial, which displays fixative general cytotoxic action against human cells, the C12-Wittig derivatives exerted their antiproliferative action mainly through cytostatic effects explaining their activity against apoptosis-resistant cancer cells. The possibility for an intriguing covalent modification of proteins through a novel pyrrole formation reaction, as well as useful activities against drug resistant cancer cells, make the described polygodial-derived chemical scaffold an interesting new chemotype warranting thorough investigation.
Keywords: Cannabidiol; Capsaicin; Glioblastoma; Ion channel; Resiniferatoxin; Vanilloid.
Copyright © 2015 Elsevier Masson SAS. All rights reserved.
Figures



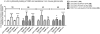

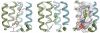


Similar articles
-
Novel polygodial analogs P3 and P27: Efficacious therapeutic agents disrupting mitochondrial function in oral squamous cell carcinoma.Int J Oncol. 2018 Dec;53(6):2627-2636. doi: 10.3892/ijo.2018.4585. Epub 2018 Oct 5. Int J Oncol. 2018. PMID: 30320372 Free PMC article.
-
Polygodial and Ophiobolin A Analogues for Covalent Crosslinking of Anticancer Targets.Int J Mol Sci. 2021 Oct 19;22(20):11256. doi: 10.3390/ijms222011256. Int J Mol Sci. 2021. PMID: 34681916 Free PMC article.
-
Synthetic and Biological Studies of Sesquiterpene Polygodial: Activity of 9-Epipolygodial against Drug-Resistant Cancer Cells.ChemMedChem. 2015 Dec;10(12):2014-26. doi: 10.1002/cmdc.201500360. Epub 2015 Oct 5. ChemMedChem. 2015. PMID: 26434977 Free PMC article.
-
Chemistry and biology of ophiobolin A and its congeners.Bioorg Med Chem Lett. 2019 Apr 1;29(7):859-869. doi: 10.1016/j.bmcl.2019.02.007. Epub 2019 Feb 7. Bioorg Med Chem Lett. 2019. PMID: 30765189 Review.
-
Pyrrole-containing hybrids as potential anticancer agents: An insight into current developments and structure-activity relationships.Eur J Med Chem. 2024 Jul 5;273:116470. doi: 10.1016/j.ejmech.2024.116470. Epub 2024 May 3. Eur J Med Chem. 2024. PMID: 38762915 Review.
Cited by
-
DNA Repair Genes ERCC1 and BRCA1 Expression in Non-Small Cell Lung Cancer Chemotherapy Drug Resistance.Med Sci Monit. 2016 Jun 12;22:1999-2005. doi: 10.12659/msm.896606. Med Sci Monit. 2016. PMID: 27289442 Free PMC article.
-
Activity of natural and synthetic polygodial derivatives against Trypanosoma cruzi amastigotes, trypomastigotes and epimastigotes.Nat Prod Res. 2021 Mar;35(5):792-795. doi: 10.1080/14786419.2019.1597350. Epub 2019 Apr 28. Nat Prod Res. 2021. PMID: 31032640 Free PMC article.
-
Novel polygodial analogs P3 and P27: Efficacious therapeutic agents disrupting mitochondrial function in oral squamous cell carcinoma.Int J Oncol. 2018 Dec;53(6):2627-2636. doi: 10.3892/ijo.2018.4585. Epub 2018 Oct 5. Int J Oncol. 2018. PMID: 30320372 Free PMC article.
-
Polygodial and Ophiobolin A Analogues for Covalent Crosslinking of Anticancer Targets.Int J Mol Sci. 2021 Oct 19;22(20):11256. doi: 10.3390/ijms222011256. Int J Mol Sci. 2021. PMID: 34681916 Free PMC article.
-
Polygodial analog induces apoptosis in LNCaP prostate cancer cells.Eur J Pharmacol. 2018 Jun 5;828:154-162. doi: 10.1016/j.ejphar.2018.03.029. Epub 2018 Mar 20. Eur J Pharmacol. 2018. PMID: 29572068 Free PMC article.
References
-
- Kaufmann SH, Earnshaw WC. Induction of apoptosis by cancer chemotherapy. Exp. Cell Res. 2000;256:42–49. - PubMed
-
- Kornienko A, Mathieu V, Rastogi S, Lefranc F, Kiss R. Therapeutic agents triggering nonapoptotic cancer cell death. J. Med. Chem. 2013;56:4823–4839. - PubMed
-
- Savage P, Stebbing J, Bower M, Crook T. Why does cytotoxic chemotherapy cure only some cancers? Nat. Clin. Pract. Oncol. 2009;6:43–52. - PubMed
-
- Wilson TR, Johnston PG, Longley DB. Anti-apoptotic mechanisms of drug resistance in cancer. Curr. Cancer Drug Targets. 2009;9:307–319. - PubMed
-
- Brenner H. Long-term survival rates of cancer patients achieved by the end of the 20th century: a period analysis. Lancet. 2002;360:1131–1135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

