Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the Pregnancy Outcome Prediction (POP) study: a prospective cohort study
- PMID: 26360240
- PMCID: PMC4655320
- DOI: 10.1016/S0140-6736(15)00131-2
Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the Pregnancy Outcome Prediction (POP) study: a prospective cohort study
Erratum in
-
Department of Error.Lancet. 2015 Nov 21;386(10008):2058. doi: 10.1016/S0140-6736(15)00976-9. Epub 2015 Nov 20. Lancet. 2015. PMID: 28831976 Free PMC article. No abstract available.
Abstract
Background: Fetal growth restriction is a major determinant of adverse perinatal outcome. Screening procedures for fetal growth restriction need to identify small babies and then differentiate between those that are healthy and those that are pathologically small. We sought to determine the diagnostic effectiveness of universal ultrasonic fetal biometry in the third trimester as a screening test for small-for-gestational-age (SGA) infants, and whether the risk of morbidity associated with being small differed in the presence or absence of ultrasonic markers of fetal growth restriction.
Methods: The Pregnancy Outcome Prediction (POP) study was a prospective cohort study of nulliparous women with a viable singleton pregnancy at the time of the dating ultrasound scan. Women participating had clinically indicated ultrasonography in the third trimester as per routine clinical care and these results were reported as usual (selective ultrasonography). Additionally, all participants had research ultrasonography, including fetal biometry at 28 and 36 weeks' gestational age. These results were not made available to participants or treating clinicians (universal ultrasonography). We regarded SGA as a birthweight of less than the 10th percentile for gestational age and screen positive for SGA an ultrasonographic estimated fetal weight of less than the 10th percentile for gestational age. Markers of fetal growth restriction included biometric ratios, utero-placental Doppler, and fetal growth velocity. We assessed outcomes for consenting participants who attended research scans and had a livebirth at the Rosie Hospital (Cambridge, UK) after the 28 weeks' research scan.
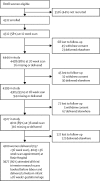
Findings: Between Jan 14, 2008, and July 31, 2012, 4512 women provided written informed consent of whom 3977 (88%) were eligible for analysis. Sensitivity for detection of SGA infants was 20% (95% CI 15-24; 69 of 352 fetuses) for selective ultrasonography and 57% (51-62; 199 of 352 fetuses) for universal ultrasonography (relative sensitivity 2·9, 95% CI 2·4-3·5, p<0·0001). Of the 3977 fetuses, 562 (14·1%) were identified by universal ultrasonography with an estimated fetal weight of less than the 10th percentile and were at an increased risk of neonatal morbidity (relative risk [RR] 1·60, 95% CI 1·22-2·09, p=0·0012). However, estimated fetal weight of less than the 10th percentile was only associated with the risk of neonatal morbidity (pinteraction=0·005) if the fetal abdominal circumference growth velocity was in the lowest decile (RR 3·9, 95% CI 1·9-8·1, p=0·0001). 172 (4%) of 3977 pregnancies had both an estimated fetal weight of less than the 10th percentile and abdominal circumference growth velocity in the lowest decile, and had a relative risk of delivering an SGA infant with neonatal morbidity of 17·6 (9·2-34·0, p<0·0001).
Interpretation: Screening of nulliparous women with universal third trimester fetal biometry roughly tripled detection of SGA infants. Combined analysis of fetal biometry and fetal growth velocity identified a subset of SGA fetuses that were at increased risk of neonatal morbidity.
Funding: National Institute for Health Research, Medical Research Council, Sands, and GE Healthcare.
Copyright © 2015 Sovio et al. Open Access article distributed under the terms of CC BY. Published by Elsevier Ltd.. All rights reserved.
Figures

Comment in
-
Should serial fetal biometry be used in all pregnancies?Lancet. 2015 Nov 21;386(10008):2038-2040. doi: 10.1016/S0140-6736(15)00148-8. Epub 2015 Sep 7. Lancet. 2015. PMID: 26360241 Free PMC article. No abstract available.
References
-
- RCOG . Green-top guideline no. 31: the investigation and management of the small-for-gestational-age fetus. Royal College of Obstetricians and Gynaecologists Press; London: 2013.
-
- American College of Obstetricians and Gynecologists ACOG practice bulletin no 101: ultrasonography in pregnancy. Obstet Gynecol. 2009;113:451–461. - PubMed
-
- NICE. National Collaborating Centre for Women's and Children's Health . NICE guideline: antenatal care. Royal College of Obstetricians and Gynaecologists Press; London: 2008.
-
- Bais JM, Eskes M, Pel M, Bonsel GJ, Bleker OP. Effectiveness of detection of intrauterine growth retardation by abdominal palpation as screening test in a low risk population: an observational study. Eur J Obstet Gynecol Reprod Biol. 2004;116:164–169. - PubMed
-
- Sparks TN, Cheng YW, McLaughlin B, Esakoff TF, Caughey AB. Fundal height: a useful screening tool for fetal growth? J Matern Fetal Neonatal Med. 2011;24:708–712. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

