Detection of Langat virus by TaqMan real-time one-step qRT-PCR method
- PMID: 26360297
- PMCID: PMC4566139
- DOI: 10.1038/srep14007
Detection of Langat virus by TaqMan real-time one-step qRT-PCR method
Abstract
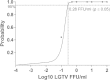
Langat virus (LGTV), one of the members of the tick-borne encephalitis virus (TBEV) complex, was firstly isolated from Ixodes granulatus ticks in Malaysia. However, the prevalence of LGTV in ticks in the region remains unknown. Surveillance for LGTV is therefore important and thus a tool for specific detection of LGTV is needed. In the present study, we developed a real-time quantitative reverse-transcription-polymerase chain reaction (qRT-PCR) for rapid detection of LGTV. Our findings showed that the developed qRT-PCR could detect LGTV at a titre as low as 0.1 FFU/ml. The detection limit of the qRT-PCR assay at 95% probability was 0.28 FFU/ml as determined by probit analysis (p ≤ 0.05). Besides, the designed primers and probe did not amplify ORF of the E genes for some closely related and more pathogenic viruses including TBEV, Louping ill virus, Omsk hemorrhagic fever virus (OHFV), Alkhurma virus (ALKV), Kyasanur Forest Disease virus (KFDV) and Powassan virus (POWV) which showed the acceptable specificity of the developed assay. The sensitivity of the developed method also has been confirmed by determining the LGTV in infected tick cell line as well as LGTV- spiked tick tissues.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
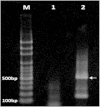
References
-
- Chambers T. J., Hahn C. S., Gailer R. & Rice C. M. Flavivirus genome organization,expression, and replication. Annu Rev Microbiol. 44, 649–688 (1990). - PubMed
-
- Smith C. G. A virus resembling Russian spring–summer encephalitis virus from an Ixodid tick in Malaya. Nature 178, 581–582 (1956). - PubMed
-
- Bancroft W. H., Scott R., Snitbhan R., Weaver R. Jr & Gould D. J. Isolation of Langat virus from Haemaphysalis papuanaThorell in Thailand. Am J Trop Med Hyg. 25, 500–504 (1976). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

