The Protein Tyrosine Phosphatase Rptpζ Suppresses Osteosarcoma Development in Trp53-Heterozygous Mice
- PMID: 26360410
- PMCID: PMC4567063
- DOI: 10.1371/journal.pone.0137745
The Protein Tyrosine Phosphatase Rptpζ Suppresses Osteosarcoma Development in Trp53-Heterozygous Mice
Abstract
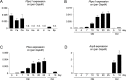
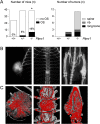
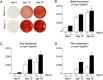
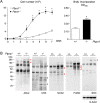
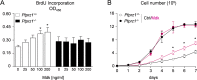
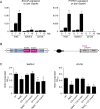
Osteosarcoma (OS), a highly aggressive primary bone tumor, belongs to the most common solid tumors in growing children. Since specific molecular targets for OS treatment remain to be identified, surgical resection combined with multimodal (neo-)adjuvant chemotherapy is still the only way to help respective individuals. We have previously identified the protein tyrosine phosphatase Rptpζ as a marker of terminally differentiated osteoblasts, which negatively regulates their proliferation in vitro. Here we have addressed the question if Rptpζ can function as a tumor suppressor protein inhibiting OS development in vivo. We therefore analyzed the skeletal phenotype of mice lacking Ptprz1, the gene encoding Rptpζ on a tumor-prone genetic background, i.e. Trp53-heterozygosity. By screening a large number of 52 week old Trp53-heterozygous mice by contact radiography we found that Ptprz1-deficiency significantly enhanced OS development with 19% of the mice being affected. The tumors in Ptprz1-deficient Trp53-heterozygous mice were present in different locations (spine, long bones, ribs), and their OS nature was confirmed by undecalcified histology. Likewise, cell lines derived from the tumors were able to undergo osteogenic differentiation ex vivo. A comparison between Ptprz1-heterozygous and Ptprz1-deficient cultures further revealed that the latter ones displayed increased proliferation, a higher abundance of tyrosine-phosphorylated proteins and resistance towards the influence of the growth factor Midkine. Our findings underscore the relevance of Rptpζ as an attenuator of proliferation in differentiated osteoblasts and raise the possibility that activating Rptpζ-dependent signaling could specifically target osteoblastic tumor cells.
Conflict of interest statement
Figures

References
-
- Bacci G, Bertoni F, Longhi A, Ferrari S, Forni C, Biagini R, et al. (2003) Neoadjuvant chemotherapy for high-grade central osteosarcoma of the extremity. Histologic response to preoperative chemotherapy correlates with histologic subtype of the tumor. Cancer 97: 3068–3075. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

