Booster dose after 10 years is recommended following 17DD-YF primary vaccination
- PMID: 26360663
- PMCID: PMC5049740
- DOI: 10.1080/21645515.2015.1082693
Booster dose after 10 years is recommended following 17DD-YF primary vaccination
Abstract
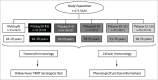
A single vaccination of Yellow Fever vaccines is believed to confer life-long protection. In this study, results of vaccinees who received a single dose of 17DD-YF immunization followed over 10 y challenge this premise. YF-neutralizing antibodies, subsets of memory T and B cells as well as cytokine-producing lymphocytes were evaluated in groups of adults before (NVday0) and after (PVday30-45, PVyear1-4, PVyear5-9, PVyear10-11, PVyear12-13) 17DD-YF primary vaccination. YF-neutralizing antibodies decrease significantly from PVyear1-4 to PVyear12-13 as compared to PVday30-45, and the seropositivity rates (PRNT≥2.9Log10mIU/mL) become critical (lower than 90%) beyond PVyear5-9. YF-specific memory phenotypes (effector T-cells and classical B-cells) significantly increase at PVday30-45 as compared to naïve baseline. Moreover, these phenotypes tend to decrease at PVyear10-11 as compared to PVday30-45. Decreasing levels of TNF-α(+) and IFN-γ(+) produced by CD4(+) and CD8(+) T-cells along with increasing levels of IL-10(+)CD4(+)T-cells were characteristic of anti-YF response over time. Systems biology profiling represented by hierarchic networks revealed that while the naïve baseline is characterized by independent micro-nets, primary vaccinees displayed an imbricate network with essential role of central and effector CD8(+) memory T-cell responses. Any putative limitations of this cross-sectional study will certainly be answered by the ongoing longitudinal population-based investigation. Overall, our data support the current Brazilian national immunization policy guidelines that recommend one booster dose 10 y after primary 17DD-YF vaccination.
Keywords: cytokine; duration of immunity; flow cytometry; memory cells and vaccination; vaccine; yellow fever.
Figures





References
-
- Monath TP. Yellow fever: an update. Lancet Infect Dis 2001; 1:11-20; PMID:11871403; http://dx.doi.org/10.1016/S1473-3099(01)00016-0 - DOI - PubMed
-
- Akondy RS, Monson ND, Miller JD, Edupuganti S, Teuwen D, Wu H, Quyyumi F, Garg S, Altman JD, Del Rio C, et al.. The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response. J Immunol 2009; 183:7919-30; PMID:19933869; http://dx.doi.org/10.4049/jimmunol.0803903 - DOI - PMC - PubMed
-
- Collaborative group for studies on yellow fever vaccines . Duration of post-vaccination immunity against yellow fever in adults. Vaccine 2014; 32:4977-84; PMID:25090646; http://dx.doi.org/10.1016/j.vaccine.2014.07.021 - DOI - PubMed
-
- Gotuzzo E, Yactayo S, Córdova E. Efficacy and duration of immunity after yellow fever vaccination: systematic review on the need for a booster every 10 years. Am J Trop Med Hyg 2013; 89:434-44; PMID:24006295; http://dx.doi.org/10.4269/ajtmh.13-0264 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
