Biofilm-Forming Abilities of Listeria monocytogenes Serotypes Isolated from Different Sources
- PMID: 26360831
- PMCID: PMC4567129
- DOI: 10.1371/journal.pone.0137046
Biofilm-Forming Abilities of Listeria monocytogenes Serotypes Isolated from Different Sources
Abstract
A total of 98 previously characterized and serotyped L. monocytogenes strains, comprising 32 of 1/2a; 20 of 1/2b and 46 of 4b serotype, from clinical and food sources were studied for their capability to form a biofilm. The microtiter plate assay revealed 62 (63.26%) strains as weak, 27 (27.55%) strains as moderate, and 9 (9.18%) strains as strong biofilm formers. Among the strong biofilm formers, 6 strains were of serotype 1/2a and 3 strains were of serotype 1/2b. None of the strain from 4b serotype exhibited strong biofilm formation. No firm correlation (p = 0.015) was noticed between any serotype and respective biofilm formation ability. Electron microscopic studies showed that strong biofilm forming isolates could synthesize a biofilm within 24 h on surfaces important in food industries such as stainless steel, ceramic tiles, high-density polyethylene plastics, polyvinyl chloride pipes, and glass. Cell enumeration of strong, moderate, and weak biofilm was performed to determine if the number of cells correlated with the biofilm-forming capabilities of the isolates. Strong, moderate, and weak biofilm showed 570±127× 103 cells/cm2, 33±26× 103 cells/cm2, 5±3× 103 cells/cm2, respectively, indicating that the number of cells was directly proportional to the strength of the biofilm. The hydrophobicity index (HI) analysis revealed higher hydrophobicity with an increased biofilm formation. Fatty acid methyl esterase analysis revealed the amount of certain fatty acids such as iso-C15:0, anteiso-C15:0, and anteiso-C17:0 fatty acids correlated with the biofilm-forming capability of L. monocytogenes. This study showed that different strains of L. monocytogenes form biofilm of different intensities which did not completely correlate with their serotype; however, it correlated with the number of cells, hydrophobicity, and amount of certain fatty acids.
Conflict of interest statement
Figures
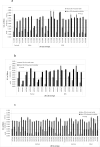


References
-
- CDC. Listeria Outbreak. Available: http://www.cdc.gov/listeria/outbreaks/index.html 24/7/14. 2014.
-
- Ramaswamy V, Cresence VM, Rejitha JS, Lekshmi MU, Dharsana KS, Prasad SP, et al. Listeria—review of epidemiology and pathogenesis. J Microbiol Immunol Infect. 2007;40: 4–13. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

