Graves' Disease Mechanisms: The Role of Stimulating, Blocking, and Cleavage Region TSH Receptor Antibodies
- PMID: 26361259
- PMCID: PMC5047290
- DOI: 10.1055/s-0035-1559633
Graves' Disease Mechanisms: The Role of Stimulating, Blocking, and Cleavage Region TSH Receptor Antibodies
Abstract
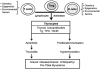
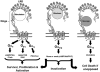
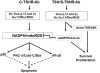
The immunologic processes involved in Graves' disease (GD) have one unique characteristic--the autoantibodies to the TSH receptor (TSHR)--which have both linear and conformational epitopes. Three types of TSHR antibodies (stimulating, blocking, and cleavage) with different functional capabilities have been described in GD patients, which induce different signaling effects varying from thyroid cell proliferation to thyroid cell death. The establishment of animal models of GD by TSHR antibody transfer or by immunization with TSHR antigen has confirmed its pathogenic role and, therefore, GD is the result of a breakdown in TSHR tolerance. Here we review some of the characteristics of TSHR antibodies with a special emphasis on new developments in our understanding of what were previously called "neutral" antibodies and which we now characterize as autoantibodies to the "cleavage" region of the TSHR ectodomain.
© Georg Thieme Verlag KG Stuttgart · New York.
Figures



References
-
- Bahn RS, Dutton CM, Natt N, Joba W, Spitzweg C, Heufelder AE. Thyrotropin receptor expression in Graves' orbital adipose/connective tissues: potential autoantigen in Graves' ophthalmopathy. J Clin Endocrinol Metab. 1998;83:998–1002. - PubMed
-
- Vlase H, Davies TF. Insights into the molecular mechanisms of the autoimmune thyroid diseases. In: Eisenbarth GS, editor. Endocrine and Organ Specific Autoimmunity. CA: R. G. Landes Co; 1999. pp. 98–132.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

