Functional Role of the Disulfide Isomerase ERp57 in Axonal Regeneration
- PMID: 26361352
- PMCID: PMC4567344
- DOI: 10.1371/journal.pone.0136620
Functional Role of the Disulfide Isomerase ERp57 in Axonal Regeneration
Erratum in
-
Correction: Functional Role of the Disulfide Isomerase ERp57 in Axonal Regeneration.PLoS One. 2015 Oct 2;10(10):e0140200. doi: 10.1371/journal.pone.0140200. eCollection 2015. PLoS One. 2015. PMID: 26431197 Free PMC article. No abstract available.
Abstract
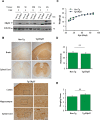
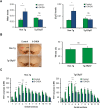
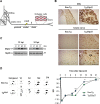
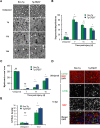
ERp57 (also known as grp58 and PDIA3) is a protein disulfide isomerase that catalyzes disulfide bonds formation of glycoproteins as part of the calnexin and calreticulin cycle. ERp57 is markedly upregulated in most common neurodegenerative diseases downstream of the endoplasmic reticulum (ER) stress response. Despite accumulating correlative evidence supporting a neuroprotective role of ERp57, the contribution of this foldase to the physiology of the nervous system remains unknown. Here we developed a transgenic mouse model that overexpresses ERp57 in the nervous system under the control of the prion promoter. We analyzed the susceptibility of ERp57 transgenic mice to undergo neurodegeneration. Unexpectedly, ERp57 overexpression did not affect dopaminergic neuron loss and striatal denervation after injection of a Parkinson's disease-inducing neurotoxin. In sharp contrast, ERp57 transgenic animals presented enhanced locomotor recovery after mechanical injury to the sciatic nerve. These protective effects were associated with enhanced myelin removal, macrophage infiltration and axonal regeneration. Our results suggest that ERp57 specifically contributes to peripheral nerve regeneration, whereas its activity is dispensable for the survival of a specific neuronal population of the central nervous system. These results demonstrate for the first time a functional role of a component of the ER proteostasis network in peripheral nerve regeneration.
Conflict of interest statement
Figures

References
-
- Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nature reviews Neuroscience. 2003;4(1):49–60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

