Effects of Exercise on Progranulin Levels and Gliosis in Progranulin-Insufficient Mice
- PMID: 26361634
- PMCID: PMC4562684
- DOI: 10.1523/ENEURO.0061-14.2015
Effects of Exercise on Progranulin Levels and Gliosis in Progranulin-Insufficient Mice
Abstract
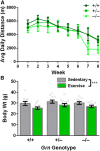
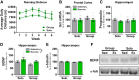
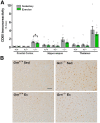
Loss-of-function mutations in progranulin (GRN) are one of the most common genetic causes of frontotemporal dementia (FTD), a progressive, fatal neurodegenerative disorder with no available disease-modifying treatments. Through haploinsufficiency, these mutations reduce levels of progranulin, a protein that has neurotrophic and anti-inflammatory effects. Increasing progranulin expression from the intact allele is therefore a potential approach for treating individuals with GRN mutations. Based on the well-known effects of physical exercise on other neurotrophic factors, we hypothesized that exercise might increase brain progranulin levels. We tested this hypothesis in progranulin heterozygous (Grn+/-) mice, which model progranulin haploinsufficiency. We housed wild-type and progranulin-insufficient mice in standard cages or cages with exercise wheels for 4 or 7.5 weeks, and then measured brain and plasma progranulin levels. Although exercise modestly increased progranulin in very young (2-month-old) wild-type mice, this effect was limited to the hippocampus. Exercise did not increase brain progranulin mRNA or protein in multiple regions, nor did it increase plasma progranulin, in 4- to 8-month-old wild-type or Grn+/- mice, across multiple experiments and under conditions that increased hippocampal BDNF and neurogenesis. Grn-/- mice were included in the study to test for progranulin-independent benefits of exercise on gliosis. Exercise attenuated cortical microgliosis in 8-month-old Grn-/- mice, consistent with a progranulin-independent, anti-inflammatory effect of exercise. These results suggest that exercise may have some modest, nonspecific benefits for FTD patients with progranulin mutations, but do not support exercise as a strategy to raise progranulin levels.
Keywords: BDNF; exercise; frontotemporal dementia; progranulin.
Conflict of interest statement
The authors report no conflict of interest.
Figures



References
-
- Ahmed Z, Sheng H, Xu YF, Lin WL, Innes AE, Gass J, Yu X, Wuertzer CA, Hou H, Chiba S, Yamanouchi K, Leissring M, Petrucelli L, Nishihara M, Hutton ML, McGowan E, Dickson DW, Lewis J (2010) Accelerated lipofuscinosis and ubiquitination in granulin knockout mice suggest a role for progranulin in successful aging. Am J Pathol 177:311–324. 10.2353/ajpath.2010.090915 - DOI - PMC - PubMed
-
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C, et al.. (2006) Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442:916–919. 10.1038/nature05016 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
