Aromatic residues in RNase T stack with nucleobases to guide the sequence-specific recognition and cleavage of nucleic acids
- PMID: 26362012
- PMCID: PMC4815224
- DOI: 10.1002/pro.2800
Aromatic residues in RNase T stack with nucleobases to guide the sequence-specific recognition and cleavage of nucleic acids
Abstract
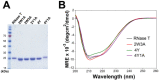
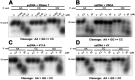
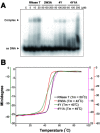
RNase T is a classical member of the DEDDh family of exonucleases with a unique sequence preference in that its 3'-to-5' exonuclease activity is blocked by a 3'-terminal dinucleotide CC in digesting both single-stranded RNA and DNA. Our previous crystal structure analysis of RNase T-DNA complexes show that four phenylalanine residues, F29, F77, F124, and F146, stack with the two 3'-terminal nucleobases. To elucidate if the π-π stacking interactions between aromatic residues and nucleobases play a critical role in sequence-specific protein-nucleic acid recognition, here we mutated two to four of the phenylalanine residues in RNase T to tryptophan (W mutants) and tyrosine (Y mutants). The Escherichia coli strains expressing either the W mutants or the Y mutants had slow growth phenotypes, suggesting that all of these mutants could not fully substitute the function of the wild-type RNase T in vivo. DNA digestion assays revealed W mutants shared similar sequence specificity with wild-type RNase T. However, the Y mutants exhibited altered sequence-dependent activity, digesting ssDNA with both 3'-end CC and GG sequences. Moreover, the W and Y mutants had reduced DNA-binding activity and lower thermal stability as compared to wild-type RNase T. Taken together, our results suggest that the four phenylalanine residues in RNase T not only play critical roles in sequence-specific recognition, but also in overall protein stability. Our results provide the first evidence showing that the π-π stacking interactions between nucleobases and protein aromatic residues may guide the sequence-specific activity for DNA and RNA enzymes.
Keywords: nucleases; protein-DNA interactions; protein-RNA interactions; π-π interactions.
© 2015 The Protein Society.
Figures
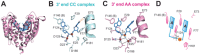

Similar articles
-
How an exonuclease decides where to stop in trimming of nucleic acids: crystal structures of RNase T-product complexes.Nucleic Acids Res. 2012 Sep;40(16):8144-54. doi: 10.1093/nar/gks548. Epub 2012 Jun 19. Nucleic Acids Res. 2012. PMID: 22718982 Free PMC article.
-
RNase R mutants elucidate the catalysis of structured RNA: RNA-binding domains select the RNAs targeted for degradation.Biochem J. 2009 Sep 25;423(2):291-301. doi: 10.1042/BJ20090839. Biochem J. 2009. PMID: 19630750
-
Characterization of the functional domains of Escherichia coli RNase II.J Mol Biol. 2006 Jul 28;360(5):921-33. doi: 10.1016/j.jmb.2006.05.043. Epub 2006 Jun 5. J Mol Biol. 2006. PMID: 16806266
-
RNase II: the finer details of the Modus operandi of a molecular killer.RNA Biol. 2010 May-Jun;7(3):276-81. doi: 10.4161/rna.7.3.11490. Epub 2010 May 9. RNA Biol. 2010. PMID: 20484980 Review.
-
Dissimilar roles of the four conserved acidic residues in the thermal stability of poly(A)-specific ribonuclease.Int J Mol Sci. 2011;12(5):2901-16. doi: 10.3390/ijms12052901. Epub 2011 May 3. Int J Mol Sci. 2011. PMID: 21686157 Free PMC article. Review.
Cited by
-
qNABpredict: Quick, accurate, and taxonomy-aware sequence-based prediction of content of nucleic acid binding amino acids.Protein Sci. 2023 Jan;32(1):e4544. doi: 10.1002/pro.4544. Protein Sci. 2023. PMID: 36519304 Free PMC article.
-
Examining tRNA 3'-ends in Escherichia coli: teamwork between CCA-adding enzyme, RNase T, and RNase R.RNA. 2018 Mar;24(3):361-370. doi: 10.1261/rna.064436.117. Epub 2017 Nov 27. RNA. 2018. PMID: 29180590 Free PMC article.
-
Structural insights into the duplex DNA processing of TREX2.Nucleic Acids Res. 2018 Dec 14;46(22):12166-12176. doi: 10.1093/nar/gky970. Nucleic Acids Res. 2018. PMID: 30357414 Free PMC article.
-
Bacterial ribonucleases and their roles in RNA metabolism.Crit Rev Biochem Mol Biol. 2019 Jun;54(3):242-300. doi: 10.1080/10409238.2019.1651816. Crit Rev Biochem Mol Biol. 2019. PMID: 31464530 Free PMC article. Review.
-
Structural basis for snRNA recognition by the double-WD40 repeat domain of Gemin5.Genes Dev. 2016 Nov 1;30(21):2391-2403. doi: 10.1101/gad.291377.116. Epub 2016 Nov 10. Genes Dev. 2016. PMID: 27881601 Free PMC article.
References
-
- Lejeune D, Delsaux N, Charloteaux B, Thomas A, Brasseur R (2005) Protein‐nucleic acid recognition: statistical analysis of atomic interactions and influence of DNA structure. Proteins 61:258–271. - PubMed
-
- Ellis JJ, Broom M, Jones S (2007) Protein‐RNA interactions: structural analysis and functional classes. Proteins 66:903–911. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

