Inhaled Cannabis for Chronic Neuropathic Pain: A Meta-analysis of Individual Patient Data
- PMID: 26362106
- PMCID: PMC4666747
- DOI: 10.1016/j.jpain.2015.07.009
Inhaled Cannabis for Chronic Neuropathic Pain: A Meta-analysis of Individual Patient Data
Abstract
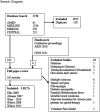
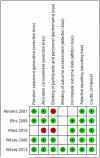
Chronic neuropathic pain, the most frequent condition affecting the peripheral nervous system, remains underdiagnosed and difficult to treat. Inhaled cannabis may alleviate chronic neuropathic pain. Our objective was to synthesize the evidence on the use of inhaled cannabis for chronic neuropathic pain. We performed a systematic review and a meta-analysis of individual patient data. We registered our protocol with PROSPERO CRD42011001182. We searched in Cochrane Central, PubMed, EMBASE, and AMED. We considered all randomized controlled trials investigating chronic painful neuropathy and comparing inhaled cannabis with placebo. We pooled treatment effects following a hierarchical random-effects Bayesian responder model for the population-averaged subject-specific effect. Our evidence synthesis of individual patient data from 178 participants with 405 observed responses in 5 randomized controlled trials following patients for days to weeks provides evidence that inhaled cannabis results in short-term reductions in chronic neuropathic pain for 1 in every 5 to 6 patients treated (number needed to treat = 5.6 with a Bayesian 95% credible interval ranging between 3.4 and 14). Our inferences were insensitive to model assumptions, priors, and parameter choices. We caution that the small number of studies and participants, the short follow-up, shortcomings in allocation concealment, and considerable attrition limit the conclusions that can be drawn from the review. The Bayes factor is 332, corresponding to a posterior probability of effect of 99.7%.
Perspective: This novel Bayesian meta-analysis of individual patient data from 5 randomized trials suggests that inhaled cannabis may provide short-term relief for 1 in 5 to 6 patients with neuropathic pain. Pragmatic trials are needed to evaluate the long-term benefits and risks of this treatment.
Keywords: Bayesian analysis; Cannabis; chronic pain; human immunodeficiency virus; meta-analysis; meta-analysis of individual patient data; neuropathy; painful; polyneuropathy.
Copyright © 2015 American Pain Society. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Abrams DI. Medical marijuana: tribulations and trials. J Psychoactive Drugs. 1998;30:163–169. - PubMed
-
- Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, Kelly ME, Rowbotham MC, Petersen KL. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515–521. - PubMed
-
- Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, Kelly ME, Rowbotham MC, Petersen KL. Cannabis in painful HIV-associated sensory neuropathy: A randomized placebo-controlled trial. Neurology. 2007;68(67):515–521. 2007. - PubMed
-
- Andreae MH, Carter G, Johnson M, Sacks H. Bayesian methods improve evidence synthesis for complementary and alternative medicine: Cannabis for painful HIV-related distal sensory polyneuropathy (HIV-DSPN) Regional Anesthesia and Pain Medicine. 2011;36:E178.
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- TL1 TR001072/TR/NCATS NIH HHS/United States
- UL1 TR000086/TR/NCATS NIH HHS/United States
- KL2 TR000088/TR/NCATS NIH HHS/United States
- 5R01AT5824/AT/NCCIH NIH HHS/United States
- TL1RR000087/RR/NCRR NIH HHS/United States
- KL2 TR001071/TR/NCATS NIH HHS/United States
- R01-AT005824/AT/NCCIH NIH HHS/United States
- KL2TR000088/TR/NCATS NIH HHS/United States
- UL1 TR001073/TR/NCATS NIH HHS/United States
- P30 MH062512/MH/NIMH NIH HHS/United States
- R01 AT005824/AT/NCCIH NIH HHS/United States
- UL1TR000086/TR/NCATS NIH HHS/United States
- 5-MO1-RR00083/RR/NCRR NIH HHS/United States
- M01 RR000083/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

