miR-132/212 deficiency impairs tau metabolism and promotes pathological aggregation in vivo
- PMID: 26362250
- PMCID: PMC4634376
- DOI: 10.1093/hmg/ddv377
miR-132/212 deficiency impairs tau metabolism and promotes pathological aggregation in vivo
Abstract
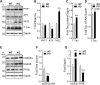
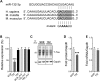
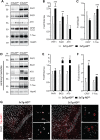
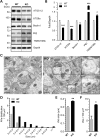
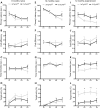
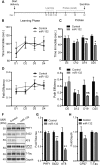
Alzheimer's disease (AD) and related tauopathies comprise a large group of neurodegenerative diseases associated with the pathological aggregation of tau protein. While much effort has focused on understanding the function of tau, little is known about the endogenous mechanisms regulating tau metabolism in vivo and how these contribute to disease. Previously, we have shown that the microRNA (miRNA) cluster miR-132/212 is downregulated in tauopathies such as AD. Here, we report that miR-132/212 deficiency in mice leads to increased tau expression, phosphorylation and aggregation. Using reporter assays and cell-based studies, we demonstrate that miR-132 directly targets tau mRNA to regulate its expression. We identified GSK-3β and PP2B as effectors of abnormal tau phosphorylation in vivo. Deletion of miR-132/212 induced tau aggregation in mice expressing endogenous or human mutant tau, an effect associated with autophagy dysfunction. Conversely, treatment of AD mice with miR-132 mimics restored in part memory function and tau metabolism. Finally, miR-132 and miR-212 levels correlated with insoluble tau and cognitive impairment in humans. These findings support a role for miR-132/212 in the regulation of tau pathology in mice and humans and provide new alternatives for therapeutic development.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

References
-
- Buee L., Bussiere T., Buee-Scherrer V., Delacourte A., Hof P.R. (2000) Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Rev., 33, 95–130. - PubMed
-
- Sergeant N., Delacourte A., Buee L. (2005) Tau protein as a differential biomarker of tauopathies. Biochimica et Biophysica Acta, 1739, 179–197. - PubMed
-
- Spillantini M.G., Goedert M. (1998) Tau protein pathology in neurodegenerative diseases. Trends Neurosci., 21, 428–433. - PubMed
-
- Tolnay M., Probst A. (1999) Review: tau protein pathology in Alzheimer's disease and related disorders. Neuropathol. Appl. Neurobiol., 25, 171–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

