The Epidemiologic and Economic Impact of Improving HIV Testing, Linkage, and Retention in Care in the United States
- PMID: 26362321
- PMCID: PMC4690480
- DOI: 10.1093/cid/civ801
The Epidemiologic and Economic Impact of Improving HIV Testing, Linkage, and Retention in Care in the United States
Abstract
Background: Recent guidelines advocate early antiretroviral therapy (ART) to decrease human immunodeficiency virus (HIV) morbidity and prevent transmission, but suboptimal engagement in care may compromise impact. We sought to determine the economic and epidemiologic impact of incomplete engagement in HIV care in the United States.
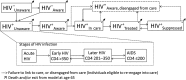
Methods: We constructed a dynamic transmission model of HIV among US adults (aged 15-65 years) and conducted a cost-effectiveness analysis of improvements along the HIV care continuum : We evaluated enhanced HIV testing (annual for high-risk groups), increased 3-month linkage to care (to 90%), and improved retention (50% relative reduction in yearly disengagement and 50% increase in reengagement). Our primary outcomes were HIV incidence, mortality, costs and quality-adjusted life-years (QALYs).
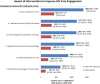
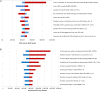
Results: Despite early ART initiation, a projected 1.39 million (95% uncertainty range [UR], 0.91-2.2 million) new HIV infections will occur at a (discounted) cost of $256 billion ($199-298 billion) over 2 decades at existing levels of HIV care engagement. Enhanced testing with increased linkage has modest epidemiologic benefits and could reduce incident HIV infections by 21% (95% UR, 13%-26%) at a cost of $65 700 per QALY gained ($44 500-111 000). By contrast, comprehensive improvements that couples enhanced testing and linkage with improved retention would reduce HIV incidence by 54% (95% UR, 37%-68%) and mortality rate by 64% (46%-78%), at a cost-effectiveness ratio of $45 300 per QALY gained ($27 800-72 300).
Conclusions: Failure to improve engagement in HIV care in the United States leads to excess infections, treatment costs, and deaths. Interventions that improve not just HIV screening but also retention in care are needed to optimize epidemiologic impact and cost-effectiveness.
Keywords: HIV; cost-effectiveness; economics; mathematical model.
© The Author 2015. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail journals.permissions@oup.com.
Figures
Comment in
-
Improving Retention in HIV Care: A Cost-effective Strategy to Turn the Tide on HIV and AIDS in the United States.Clin Infect Dis. 2016 Jan 15;62(2):230-2. doi: 10.1093/cid/civ804. Epub 2015 Sep 11. Clin Infect Dis. 2016. PMID: 26362322 No abstract available.
References
-
- DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents, 2013. Available at: http://aidsinfo.nih.gov/guidelines Accessed 15 January 2014.
-
- Quinn TC, Wawer MJ, Sewankambo N et al. . Rakai Project Study Group. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med 2000; 342:921–9. - PubMed
-
- Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet 2009; 373:48–57. - PubMed
-
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs fro treating and preventing HIV infection. Available at: http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf?ua=1 Accessed 5 June 2014.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

