Arabidopsis SEIPIN Proteins Modulate Triacylglycerol Accumulation and Influence Lipid Droplet Proliferation
- PMID: 26362606
- PMCID: PMC4815042
- DOI: 10.1105/tpc.15.00588
Arabidopsis SEIPIN Proteins Modulate Triacylglycerol Accumulation and Influence Lipid Droplet Proliferation
Abstract
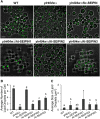
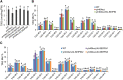
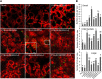
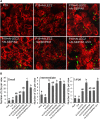
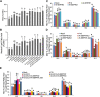
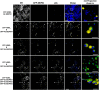
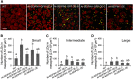
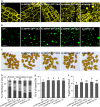
The lipodystrophy protein SEIPIN is important for lipid droplet (LD) biogenesis in human and yeast cells. In contrast with the single SEIPIN genes in humans and yeast, there are three SEIPIN homologs in Arabidopsis thaliana, designated SEIPIN1, SEIPIN2, and SEIPIN3. Essentially nothing is known about the functions of SEIPIN homologs in plants. Here, a yeast (Saccharomyces cerevisiae) SEIPIN deletion mutant strain and a plant (Nicotiana benthamiana) transient expression system were used to test the ability of Arabidopsis SEIPINs to influence LD morphology. In both species, expression of SEIPIN1 promoted accumulation of large-sized lipid droplets, while expression of SEIPIN2 and especially SEIPIN3 promoted small LDs. Arabidopsis SEIPINs increased triacylglycerol levels and altered composition. In tobacco, endoplasmic reticulum (ER)-localized SEIPINs reorganized the normal, reticulated ER structure into discrete ER domains that colocalized with LDs. N-terminal deletions and swapping experiments of SEIPIN1 and 3 revealed that this region of SEIPIN determines LD size. Ectopic overexpression of SEIPIN1 in Arabidopsis resulted in increased numbers of large LDs in leaves, as well as in seeds, and increased seed oil content by up to 10% over wild-type seeds. By contrast, RNAi suppression of SEIPIN1 resulted in smaller seeds and, as a consequence, a reduction in the amount of oil per seed compared with the wild type. Overall, our results indicate that Arabidopsis SEIPINs are part of a conserved LD biogenesis machinery in eukaryotes and that in plants these proteins may have evolved specialized roles in the storage of neutral lipids by differentially modulating the number and sizes of lipid droplets.
© 2015 American Society of Plant Biologists. All rights reserved.
Figures



References
-
- Andrianov V., Borisjuk N., Pogrebnyak N., Brinker A., Dixon J., Spitsin S., Flynn J., Matyszczuk P., Andryszak K., Laurelli M., Golovkin M., Koprowski H. (2010). Tobacco as a production platform for biofuel: overexpression of Arabidopsis DGAT and LEC2 genes increases accumulation and shifts the composition of lipids in green biomass. Plant Biotechnol. J. 8: 277–287. - PubMed
-
- Bartz R., Li W.H., Venables B., Zehmer J.K., Roth M.R., Welti R., Anderson R.G., Liu P., Chapman K.D. (2007). Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J. Lipid Res. 48: 837–847. - PubMed
-
- Baud S., Lepiniec L. (2010). Physiological and developmental regulation of seed oil production. Prog. Lipid Res. 49: 235–249. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

