Effect of zinc added to a daily small-quantity lipid-based nutrient supplement on diarrhoea, malaria, fever and respiratory infections in young children in rural Burkina Faso: a cluster-randomised trial
- PMID: 26362661
- PMCID: PMC4567679
- DOI: 10.1136/bmjopen-2015-007828
Effect of zinc added to a daily small-quantity lipid-based nutrient supplement on diarrhoea, malaria, fever and respiratory infections in young children in rural Burkina Faso: a cluster-randomised trial
Abstract
Objective: Preventive zinc supplementation in the form of tablets or syrup reduces the incidence of diarrhoea and acute lower respiratory tract infections (RTI), but its effect on malaria is inconsistent. When zinc is administered with other micronutrients or foods, its effect is also uncertain. We assessed the effects of different amounts and sources of zinc on the frequency of diarrhoea, malaria, fever and RTI in young children.
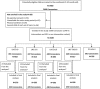
Design, setting and populations: This community-based, double-blind, placebo-controlled, cluster-randomised trial of 2435 children 9 months of age was carried out between April 2010 and July 2012 in rural southwestern Burkina Faso.
Interventions: Participants were randomly assigned at the concession level to receive daily 1 of 4 interventions for 9 months: (1) 20 g small-quantity lipid-based nutrient supplement (SQ-LNS) without zinc and placebo tablet, (2) 20 g SQ-LNS with 5 mg zinc and placebo tablet, (3) 20 g SQ-LNS with 10 mg zinc and placebo tablet or (4) 20 g SQ-LNS without zinc and 5 mg zinc tablet. Participants were visited weekly in their homes for morbidity surveillance for 9 months, and those with uncomplicated diarrhoea and malaria received treatment from the study field workers in the community.
Main outcomes: Incidence and longitudinal prevalence of diarrhoea, malaria, fever, and lower and upper RTI by intervention group.
Results: The incidence of diarrhoea, malaria and fever was 1.10 (±1.03 SD), 0.61 (±0.66 SD) and 1.49 (±1.12 SD) episodes per 100 child-days at risk, respectively, and did not differ by intervention group (p=0.589, p=0.856 and p=0.830, respectively). The longitudinal prevalence of acute lower RTI (0.1%; 95% IC 0.1-0.2%) and of upper RTI (7.8%; 95% IC 7.1-8.4%) did not differ among groups (p=0.234 and p=0.501, respectively).
Conclusions: Inclusion of 5 or 10 mg zinc in SQ-LNS and provision of 5 mg zinc dispersible tablet along with SQ-LNS had no impact on the incidence of diarrhoea, malaria and fever or the longitudinal prevalence of RTI compared with SQ-LNS without zinc in this population.
Trial registration number: NCT00944281.
Keywords: NUTRITION & DIETETICS.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
References
-
- Brown KH, Peerson JM, Baker SK et al. . Preventive zinc supplementation among infants, preschoolers, and older prepubertal children. Food Nutr Bull 2009;30(Suppl 1):S12–40. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
