Hippocampal PPARδ Overexpression or Activation Represses Stress-Induced Depressive Behaviors and Enhances Neurogenesis
- PMID: 26362775
- PMCID: PMC4772271
- DOI: 10.1093/ijnp/pyv083
Hippocampal PPARδ Overexpression or Activation Represses Stress-Induced Depressive Behaviors and Enhances Neurogenesis
Erratum in
-
Erratum.Int J Neuropsychopharmacol. 2016 Apr 27;19(10):pyw031. doi: 10.1093/ijnp/pyw031. Int J Neuropsychopharmacol. 2016. PMID: 27207904 Free PMC article. No abstract available.
Abstract
Background: Emerging data have demonstrated that peroxisome proliferator-activated receptor δ (PPARδ) activation confers a potentially neuroprotective role in some neurodegenerative diseases. However, whether PPARδ is involved in depression is unknown.
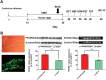
Methods: In this study, PPARδ was firstly investigated in the chronic mild stress (CMS) and learned helplessness (LH) models of depression. The changes in depressive behaviors and hippocampal neurogenesis were investigated after PPARδ overexpression by microinfusion of the lentiviral vector, containing the coding sequence of mouse PPARδ (LV-PPARδ), into the bilateral dentate gyri of the hippocampus or PPARδ activation by repeated systemic administration of PPARδ agonist GW0742 (5 or 10mg/kg.d, i.p., for 21 d).
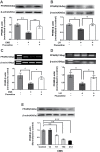
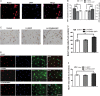
Results: We found that both CMS and LH resulted in a significant decrease in the PPARδ expression in the hippocampi of mice, and this change was reversed by treatment with the antidepressant fluoxetine. PPARδ overexpression and PPARδ activation each suppressed the CMS- and LH-induced depressive-like behavior and produced an antidepressive effect. In vivo or in vitro studies also showed that both overexpression and activation of PPARδ enhanced proliferation or differentiation of neural stem cells in the hippocampi of mice.
Conclusions: These results suggest that hippocampal PPARδ upregulation represses stress-induced depressive behaviors, accompanied by enhancement of neurogenesis.
Keywords: Depression; hippocampal neurogenesis; peroxisome proliferator-activated receptors δ.
© The Author 2015. Published by Oxford University Press on behalf of CINP.
Figures




References
-
- Arsenijevic D, de Bilbao F, Plamondon J, Paradis E, Vallet P, Richard D, Lanqhans W, Giannakopoulos P. (2006) Increased infarct size and lack of hyperphagic response after focal cerebral ischemia in peroxisome proliferator-activated receptor beta-deficient mice. J Cereb Blood Flow Metab 26:433–445. - PubMed
-
- Belmaker RH, Agam G. (2008) Major depressive disorder. N Engl J Med 358:55–68. - PubMed
-
- Braissant O, Foufelle F, Scotto C, Dauça M, Wahli W. (1996) Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology 137:354–366. - PubMed
-
- Caldarone BJ, George TP, Zachariou V, Picciotto MR. (2000) Gender differences in learned helplessness behavior are influenced by genetic background. Pharmacol Biochem Behav 66:811–817. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

