NK Cells Preferentially Target Tumor Cells with a Cancer Stem Cell Phenotype
- PMID: 26363055
- PMCID: PMC4781667
- DOI: 10.4049/jimmunol.1500447
NK Cells Preferentially Target Tumor Cells with a Cancer Stem Cell Phenotype
Abstract
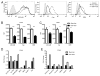
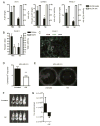
Increasing evidence supports the hypothesis that cancer stem cells (CSCs) are resistant to antiproliferative therapies, able to repopulate tumor bulk, and seed metastasis. NK cells are able to target stem cells as shown by their ability to reject allogeneic hematopoietic stem cells but not solid tissue grafts. Using multiple preclinical models, including NK coculture (autologous and allogeneic) with multiple human cancer cell lines and dissociated primary cancer specimens and NK transfer in NSG mice harboring orthotopic pancreatic cancer xenografts, we assessed CSC viability, CSC frequency, expression of death receptor ligands, and tumor burden. We demonstrate that activated NK cells are capable of preferentially killing CSCs identified by multiple CSC markers (CD24(+)/CD44(+), CD133(+), and aldehyde dehydrogenase(bright)) from a wide variety of human cancer cell lines in vitro and dissociated primary cancer specimens ex vivo. We observed comparable effector function of allogeneic and autologous NK cells. We also observed preferential upregulation of NK activation ligands MICA/B, Fas, and DR5 on CSCs. Blocking studies further implicated an NKG2D-dependent mechanism for NK killing of CSCs. Treatment of orthotopic human pancreatic cancer tumor-bearing NSG mice with activated NK cells led to significant reductions in both intratumoral CSCs and tumor burden. Taken together, these data from multiple preclinical models, including a strong reliance on primary human cancer specimens, provide compelling preclinical evidence that activated NK cells preferentially target cancer cells with a CSC phenotype, highlighting the translational potential of NK immunotherapy as part of a combined modality approach for refractory solid malignancies.
Copyright © 2015 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures




References
-
- Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10:717–728. - PubMed
-
- Valent P, Bonnet D, De Maria R, Lapidot T, Copland M, Melo JV, Chomienne C, Ishikawa F, Schuringa JJ, Stassi G, et al. Cancer stem cell definitions and terminology: the devil is in the details. Nat Rev Cancer. 2012;12:767–775. - PubMed
-
- Wang L, Park P, Zhang H, La Marca F, Lin CY. Prospective identification of tumorigenic osteosarcoma cancer stem cells in OS99-1 cells based on high aldehyde dehydrogenase activity. Int J Cancer. 2011;128:294–303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

