Long term survival with cytotoxic T lymphocyte-associated antigen 4 blockade using tremelimumab
- PMID: 26364516
- PMCID: PMC4821004
- DOI: 10.1016/j.ejca.2015.08.012
Long term survival with cytotoxic T lymphocyte-associated antigen 4 blockade using tremelimumab
Abstract
Purpose: One of the hallmarks of cancer immunotherapy is the long duration of responses, evident with cytokines like interleukin-2 or a variety of cancer vaccines. However, there is limited information available on very long term outcomes of patients treated with anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) antibodies. Tremelimumab is an anti-CTLA-4 antibody of immunoglobulin G2 (IgG2) isotype initially tested in patients with advanced melanoma over 12 years ago.
Methods: We reviewed the outcomes of patients with advanced melanoma enrolled in four phase 1 and 2 tremelimumab trials at two sites to determine response rates and long-term survival.
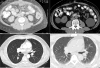
Results: A total of 143 patients were enrolled at two institutions from 2002 to 2008. Tremelimumab administration varied between a single dose of 0.01 mg/kg and 15 mg/kg every 3 months. Median overall survival was 13 months (95% confidence interval (CI), 10-16.6), ranging from less than a month to 12+ years. An objective response rate of 15.6% was observed, with median duration of response of 6.5 years, range of 3-136+ months. The Kaplan-Meier estimated 5 year survival rate was 20% (95% CI, 13-26%), with 10 and 12.5 year survival rates of 16% (95% CI, 9-23%).
Conclusions: CTLA-4 blockade with tremelimumab can lead to very long duration of objective anti-tumour responses beyond 12 years.
Keywords: Anti-CTLA-4 therapy; Immunotherapy; Melanoma; Tremelimumab.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures


Comment in
-
How should we use anti-CTLA-4 antibodies?Eur J Cancer. 2015 Nov;51(17):2686-8. doi: 10.1016/j.ejca.2015.09.002. Eur J Cancer. 2015. PMID: 26613660 No abstract available.
References
-
- Kaufman HL, A R, Collichio FA, et al. Primary overall survival (OS) from OPTiM, a randomized phase III trial of talimogene laherparepvec (T-VEC) versus subcutaneous (SC) granulocyte-macrophage colony-stimulating factor (GM-CSF) for the treatment (tx) of unresected stage IIIB/C and IV melanoma. J Clin Oncol. 2014;32:5s. (suppl; abstr 9008a)
-
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–22. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

