YAP induces high-grade serous carcinoma in fallopian tube secretory epithelial cells
- PMID: 26364602
- PMCID: PMC4791205
- DOI: 10.1038/onc.2015.288
YAP induces high-grade serous carcinoma in fallopian tube secretory epithelial cells
Abstract
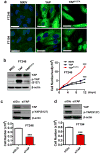
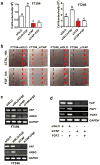
Accumulating evidence indicates that ovarian high-grade serous carcinoma (HGSC) originates from fallopian tube secretory epithelial cells (FTSECs). However, the molecular mechanisms underlying the initiation and progression of HGSC derived from FTSECs remains unclear. In this study, we found that the Hippo/Yes-associated protein (YAP) signaling pathway has a critical role in the initiation and progression of fallopian tube and ovarian HGSC. Importantly, YAP was overexpressed in inflammatory and cancerous fallopian tube tissues. Further, overexpression of wild-type YAP, or constitutively active YAP in immortalized FTSECs, induced cell proliferation, migration, colony formation and tumorigenesis. Moreover, the Hippo/YAP and the fibroblast growth factor (FGF) signaling pathways formed an autocrine/paracrine-positive feedback loop to drive the progression of the FTSEC-derived HGSC. Evidence in this study strongly suggests that combined therapy with inhibitors of YAP (such as verteporfin) and FGF receptors (such as BGJ398) can provide a novel therapeutic strategy to treat fallopian tube and ovarian HGSC.
Conflict of interest statement
Figures









References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Seidman JD, Horkayne-Szakaly I, Haiba M, Boice CR, Kurman RJ, Ronnett BM. The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int J Gynecol Pathol. 2004;23:41–44. - PubMed
-
- Selvaggi SM. Tumors of the ovary, maldeveloped gonads, fallopian tube, and broad ligament. Arch Pathol Lab Med. 2000;124:477. - PubMed
-
- Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev. 2001;22:255–288. - PubMed
-
- Katabuchi H, Okamura H. Cell biology of human ovarian surface epithelial cells and ovarian carcinogenesis. Med Electron Microsc. 2003;36:74–86. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

