Experimental evaluation of the zoonotic infection potency of simian retrovirus type 4 using humanized mouse model
- PMID: 26364986
- PMCID: PMC4568461
- DOI: 10.1038/srep14040
Experimental evaluation of the zoonotic infection potency of simian retrovirus type 4 using humanized mouse model
Abstract
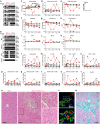
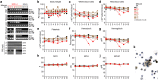
During 2001-2002 and 2008-2011, two epidemic outbreaks of infectious hemorrhagic disease have been found in Japanese macaques (Macaca fuscata) in Kyoto University Primate Research Institute, Japan. Following investigations revealed that the causative agent was simian retrovirus type 4 (SRV-4). SRV-4 was isolated by using human cell lines, which indicates that human cells are potently susceptible to SRV-4 infection. These raise a possibility of zoonotic infection of pathogenic SRV-4 from Japanese macaques into humans. To explore the possibility of zoonotic infection of SRV-4 to humans, here we use a human hematopoietic stem cell-transplanted humanized mouse model. Eight out of the twelve SRV-4-inoculated humanized mice were infected with SRV-4. Importantly, 3 out of the 8 infected mice exhibited anemia and hemophagocytosis, and an infected mouse died. To address the possibility that SRV-4 adapts humanized mouse and acquires higher pathogenicity, the virus was isolated from an infected mice exhibited severe anemia was further inoculated into another 6 humanized mice. However, no infected mice exhibited any illness. Taken together, our findings demonstrate that the zoonotic SRV-4 infection from Japanese macaques to humans is technically possible under experimental condition. However, such zoonotic infection may not occur in the real society.
Figures
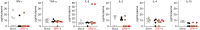


References
-
- Daniel M. D. et al.. A new type D retrovirus isolated from macaques with an immunodeficiency syndrome. Science 223, 602–605 (1984). - PubMed
-
- Marx P. A. et al.. Simian AIDS: isolation of a type D retrovirus and transmission of the disease. Science 223, 1083–1086 (1984). - PubMed
-
- Montiel N. A. An updated review of simian betaretrovirus (SRV) in macaque hosts. J. Med. Primatol. 39, 303–314 (2010). - PubMed
-
- Henrickson R. V. et al.. Clinical features of simian acquired immunodeficiency syndrome (SAIDS) in rhesus monkeys. Lab. Anim. Sci. 34, 140–145 (1984). - PubMed
-
- Committee on Disease Control, Primate Research Institute & University, K. Information of Hemorrhagic Syndrome of Japanese Macaques (Provisional Designation). Primate Research 26, 69–71 10.2354/psj.2326.2369 (2010). - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

